MARKET OVERVIEW
The China Intracranial Pressure Monitoring Devices market denotes a burgeoning sector within the medical device industry, specifically tailored to the intricate realm of neurology. This industry encapsulates a spectrum of devices meticulously designed to measure and manage intracranial pressure (ICP), a critical parameter in monitoring neurological conditions such as traumatic brain injuries, intracranial hemorrhages, and hydrocephalus. As China's healthcare infrastructure continues to evolve, propelled by advancements in technology and growing awareness of neurological disorders, the demand for innovative intracranial pressure monitoring devices is poised to witness a substantial surge.
The landscape of the China Intracranial Pressure Monitoring Devices market is characterized by a myriad of players, including multinational corporations and domestic manufacturers, vying for market share. These entities invest heavily in research and development to introduce cutting-edge devices that cater to the specific needs of healthcare professionals and patients alike. Additionally, stringent regulatory standards and quality assurance protocols ensure the safety and efficacy of these devices, bolstering consumer confidence in the market.
The primary objective of intracranial pressure monitoring devices is to provide clinicians with real-time data on intracranial pressure dynamics, enabling timely intervention and personalized patient management strategies. These devices employ various techniques, such as invasive catheter-based systems, non-invasive methods, and advanced imaging modalities, to accurately assess intracranial pressure levels. Moreover, the integration of wireless technology and cloud-based platforms facilitates seamless data transmission and remote monitoring, enhancing the accessibility and efficiency of patient care.
In the foreseeable future, the China Intracranial Pressure Monitoring Devices market is poised to witness a paradigm shift towards miniaturization and portability, driven by the increasing demand for point-of-care diagnostics and ambulatory monitoring solutions. Furthermore, advancements in sensor technology and data analytics are anticipated to revolutionize intracranial pressure monitoring, enabling predictive modeling and personalized treatment algorithms tailored to individual patient profiles
The burgeoning prevalence of neurological disorders, coupled with the aging population and rising healthcare expenditures, underscores the immense growth potential of the China Intracranial Pressure Monitoring Devices market. As healthcare systems strive to optimize patient outcomes while containing costs, the adoption of intracranial pressure monitoring devices is projected to become ubiquitous across neurocritical care units, emergency departments, and ambulatory settings.
The China Intracranial Pressure Monitoring Devices market represents a dynamic and rapidly evolving industry at the nexus of healthcare and technology. With a diverse array of innovative devices and technologies poised to reshape the landscape of neurological care, this market holds immense promise in improving patient outcomes and advancing the frontiers of neurology in the years to come.
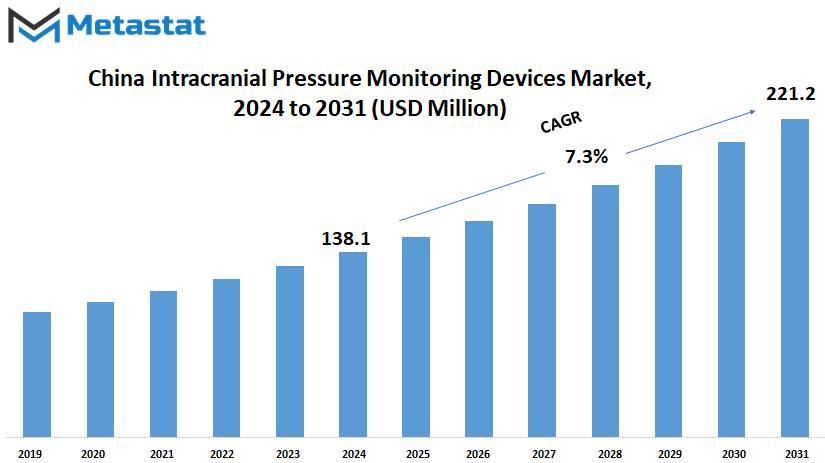
China Intracranial Pressure Monitoring Devices market is estimated to reach $221.2 Million by 2031; growing at a CAGR of 7.3% from 2024 to 2031.
GROWTH FACTORS
The China market for Intracranial Pressure Monitoring Devices is witnessing notable growth, primarily fueled by the increasing prevalence of multidrug-resistant bacterial infections. This surge in infections demands potent antibiotics like carbapenems for effective treatment. Furthermore, the rising incidence of healthcare-associated infections and complicated intra-abdominal infections further accentuates the necessity for such antibiotics. However, challenges loom over this growth trajectory. The emergence of carbapenem-resistant bacteria poses a significant threat, resulting in treatment failures and reducing the effectiveness of carbapenem therapy. Additionally, stringent regulatory requirements and safety concerns surrounding the use of carbapenem antibiotics impose restrictions on their prescription and usage, potentially hindering market expansion.
Despite these challenges, the market outlook is not bleak. Opportunities for growth and innovation persist, especially with the development of novel carbapenem formulations or combination therapies. These advancements aim to overcome resistance mechanisms and enhance antibacterial efficacy, thereby revitalizing market prospects and fostering innovation in antibiotic development. Such initiatives are poised to unlock lucrative opportunities in the coming years, driving market expansion and addressing the evolving needs of healthcare systems grappling with antimicrobial resistance.
The China Intracranial Pressure Monitoring Devices market is navigating a dynamic landscape shaped by both challenges and opportunities. While the specter of carbapenem-resistant bacteria and regulatory hurdles cast shadows over growth prospects, the pursuit of innovative solutions holds promise for overcoming these obstacles. As stakeholders continue to adapt and innovate, the market is poised to chart a resilient path forward, bolstered by a commitment to combating infectious diseases and advancing patient care.
MARKET SEGMENTATION
By Product Type
In the realm of medical devices, especially those concerning brain health, the market for Intracranial Pressure Monitoring Devices in China is witnessing significant growth. These devices play a crucial role in monitoring and managing conditions affecting the brain, such as traumatic brain injuries, strokes, and certain neurological disorders.
One of the key aspects of this market is the segmentation based on product types, which include invasive and non-invasive devices. Invasive devices involve the insertion of a sensor or probe directly into the brain tissue or the cerebral spinal fluid, allowing for precise and continuous monitoring of intracranial pressure. On the other hand, non-invasive devices offer a less invasive approach, typically utilizing external sensors placed on the scalp or within the skull to measure intracranial pressure indirectly.
The demand for both types of devices is expected to increase in the coming years, driven by several factors. Firstly, the rising incidence of traumatic brain injuries due to accidents, sports-related injuries, and other causes necessitates better methods for monitoring and managing intracranial pressure. Additionally, the growing prevalence of neurological disorders, such as hydrocephalus and intracranial hemorrhage, further fuels the demand for these devices.
Furthermore, advancements in technology are driving innovation in the field of intracranial pressure monitoring devices. Manufacturers are constantly striving to develop devices that are more accurate, reliable, and user-friendly. This includes the integration of wireless connectivity and cloud-based platforms for remote monitoring and data analysis, enhancing the overall efficiency and effectiveness of these devices.
Moreover, increased healthcare spending and infrastructure development in China are creating favorable conditions for market growth. As the healthcare system continues to modernize and expand, there will be greater access to advanced medical technologies, including intracranial pressure monitoring devices.
The China Intracranial Pressure Monitoring Devices market is poised for significant growth, driven by factors such as the rising incidence of brain-related conditions, technological advancements, and improving healthcare infrastructure. With both invasive and non-invasive devices playing a vital role in patient care, manufacturers have ample opportunities to innovate and cater to the evolving needs of healthcare providers and patients alike. By Route of Intervention the market is divided into Intraventricular, Subdural, Epidural, Parenchymal, Subarachnoid.
By Indication
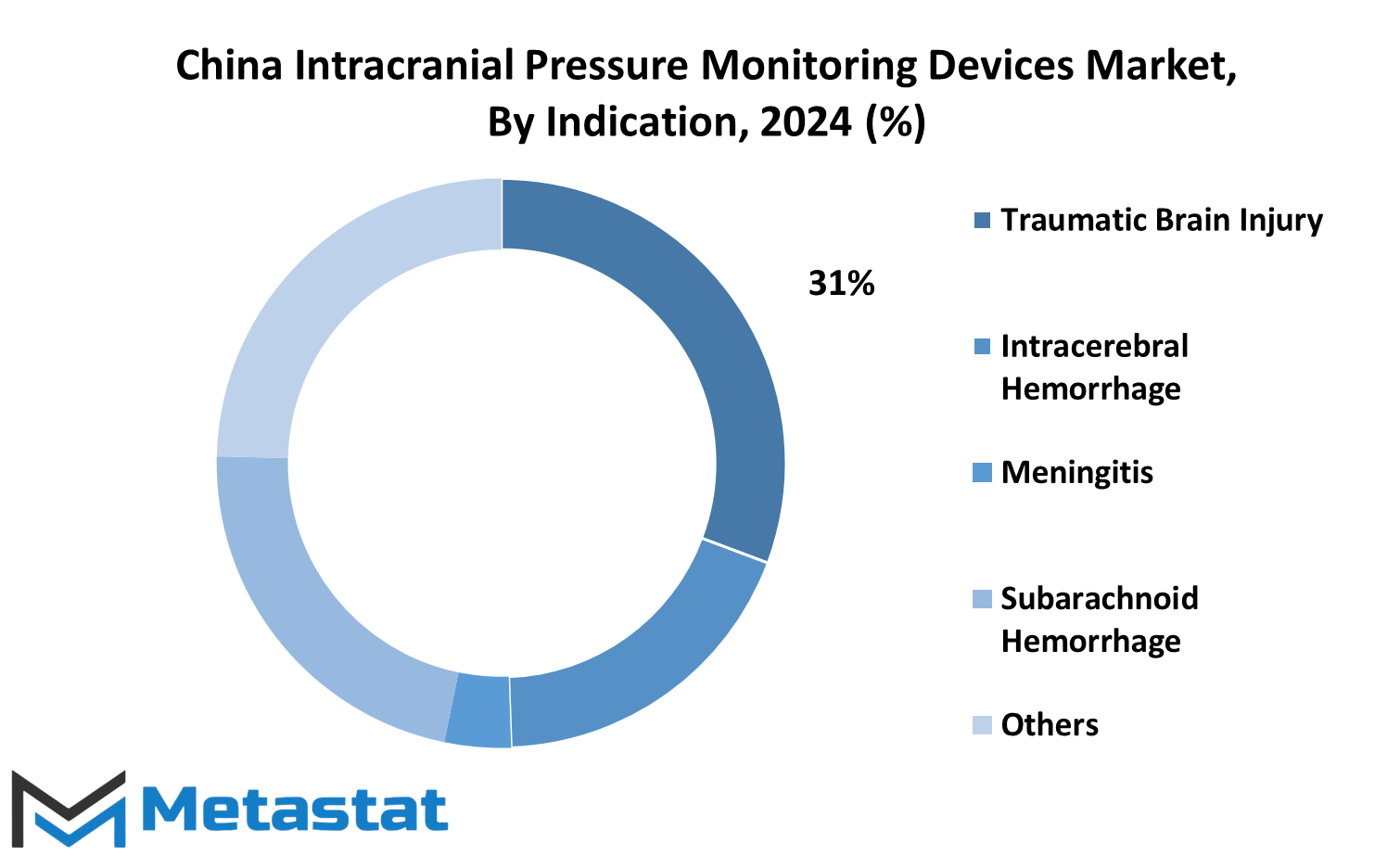
The market for intracranial pressure monitoring devices in China is experiencing notable growth, driven by various indications. These indications include traumatic brain injury, intracerebral hemorrhage, subarachnoid hemorrhage, hydrocephalus, malignant cerebral infarction (MCI), cerebral edema, CNS infections, and others.
In 2021, the traumatic brain injury segment accounted for a significant portion of this market, valued at 35 million USD. Traumatic brain injuries, often resulting from severe blows or jolts to the head, can lead to increased intracranial pressure, necessitating monitoring and management.
Similarly, the intracerebral hemorrhage segment contributed substantially to the market, with a value of 21.1 million USD in 2021. Intracerebral hemorrhage refers to bleeding within the brain tissue itself, which can elevate intracranial pressure and require monitoring to prevent further complications.
Other indications such as subarachnoid hemorrhage, hydrocephalus, malignant cerebral infarction, cerebral edema, CNS infections, and miscellaneous conditions also play essential roles in driving the demand for intracranial pressure monitoring devices in China. Each of these conditions presents unique challenges in managing intracranial pressure, making accurate monitoring crucial for patient care.
Moreover, ongoing efforts to improve healthcare infrastructure and access to advanced medical devices across China will enhance market penetration and drive adoption rates. This expansion will likely be supported by government initiatives aimed at promoting healthcare innovation and improving patient outcomes.
Furthermore, the increasing focus on personalized medicine and tailored treatment approaches will drive the development of intracranial pressure monitoring devices that are more user-friendly, efficient, and customizable to individual patient needs. Such innovations will not only improve patient care but also contribute to the overall growth and advancement of the healthcare sector in China.
The market for intracranial pressure monitoring devices in China is experiencing significant growth, driven by various neurological indications. With ongoing technological advancements and supportive government policies, the future outlook for this market is promising, with opportunities for further expansion and innovation.
By End User
The market for Intracranial Pressure Monitoring Devices in China is segmented based on end users, including Hospitals, Ambulatory Surgical Centers, Trauma Centers, and Neurosurgery Centers. These segments will help us understand how different institutions utilize these devices and what their specific needs and preferences are.
Hospitals, being the primary healthcare providers, will likely be the largest consumer of these devices. They will use them to monitor patients with various neurological conditions, such as traumatic brain injuries, strokes, or brain tumors. Hospitals handle a wide range of cases and require versatile monitoring devices to cater to diverse patient needs.
Ambulatory Surgical Centers, on the other hand, specialize in providing same-day surgical care without the need for overnight hospitalization. These centers may require fewer monitoring devices compared to hospitals, but they still need them for certain surgical procedures that carry a risk of increased intracranial pressure.
Trauma Centers play a crucial role in managing severe injuries, including those to the head and brain. In these facilities, rapid and accurate monitoring of intracranial pressure can be a matter of life and death. Therefore, they will likely invest in high-quality monitoring devices to ensure timely interventions for patients in critical condition.
Neurosurgery Centers focus specifically on surgical interventions for neurological conditions. These centers will heavily rely on intracranial pressure monitoring devices not only during surgeries but also in the post-operative period to assess patients' recovery and detect any complications early on.
Each of these end-user segments will have unique requirements and preferences when it comes to intracranial pressure monitoring devices. Hospitals may prioritize devices that are easy to use and integrate into their existing healthcare infrastructure. Ambulatory Surgical Centers may value portability and convenience, as they often perform procedures in outpatient settings. Trauma Centers will prioritize devices that offer real-time monitoring capabilities and are robust enough to withstand the rigors of emergency care. Neurosurgery Centers, being specialized facilities, may seek devices with advanced features and precise measurements to support complex surgical procedures and post-operative care.
Understanding the different end-user segments in the China Intracranial Pressure Monitoring Devices market is crucial for manufacturers and suppliers to tailor their products and services effectively. By catering to the specific needs and preferences of hospitals, ambulatory surgical centers, trauma centers, and neurosurgery centers, they can ensure the widespread adoption and optimal utilization of these critical medical devices across various healthcare settings.
COMPETITIVE PLAYERS
The Chinese market for Intracranial Pressure Monitoring Devices is witnessing significant growth, driven by key players like Medtronic, Integra LifeSciences, RAUMEDIC, Sophysa, Spiegelberg, Natus Medical, HAIYING MEDICAL, Camino Laboratories, Jiangsu Yuyue Medical Technology Co., Ltd., and Haiying Medical Technology Co., Ltd. These companies play a pivotal role in shaping the landscape of the Intracranial Pressure Monitoring Devices industry in China.
As technology advances, these key players will continue to innovate, developing cutting-edge devices that enhance patient care and outcomes. Medtronic, a leader in medical technology, will likely introduce new features and functionalities in their monitoring devices, setting new standards for the industry. Similarly, Integra LifeSciences, known for its neurosurgical innovations, will contribute to the market with its expertise in intracranial pressure monitoring.
RAUMEDIC, a global manufacturer of medical devices, is expected to leverage its experience and resources to expand its presence in the Chinese market. Sophysa, with its focus on neurosurgery and hydrocephalus management, will likely introduce advanced monitoring solutions tailored to the specific needs of Chinese healthcare providers.
Spiegelberg, a pioneer in intracranial pressure measurement, will continue to refine its devices, ensuring accuracy and reliability in critical care settings. Natus Medical, specializing in neurology and newborn care, will play a vital role in meeting the growing demand for intracranial pressure monitoring devices in China.
HAIYING MEDICAL and Haiying Medical Technology Co., Ltd., local players with a deep understanding of the Chinese market, will contribute to the industry by offering cost-effective solutions without compromising on quality. Camino Laboratories, known for its advanced neurosurgical products, will likely introduce innovative monitoring devices that address the unique challenges faced by healthcare providers in China.
Jiangsu Yuyue Medical Technology Co., Ltd., a leading manufacturer of medical equipment, will continue to invest in research and development, driving advancements in intracranial pressure monitoring technology. Their contributions will be crucial in improving patient outcomes and enhancing the overall quality of care in China.
The Intracranial Pressure Monitoring Devices market in China is poised for significant growth, with key players like Medtronic, Integra LifeSciences, RAUMEDIC, Sophysa, Spiegelberg, Natus Medical, HAIYING MEDICAL, Camino Laboratories, Jiangsu Yuyue Medical Technology Co., Ltd., and Haiying Medical Technology Co., Ltd. driving innovation and shaping the future of healthcare in the region.
China Intracranial Pressure Monitoring Devices Market Key Segments:
By Product Type
- Invasive
- Non-invasive
By Route of Intervention
- Intraventricular
- Subdural
- Epidural
- Parenchymal
- Subarachnoid
By Indication
- Traumatic Brain Injury
- Intracerebral Hemorrhage
- Subarachnoid Hemorrhage
- Hydrocephalus
- Malignant Cerebral Infarction (MCI)
- Cerebral Edema
- CNS Infections
- Others
By End User
- Hospitals
- Ambulatory Surgical Centers
- Trauma Centers
- Neurosurgery Centers
Key China Intracranial Pressure Monitoring Devices Industry Players
- Medtronic
- Integra LifeSciences
- RAUMEDIC
- Sophysa
- Spiegelberg
- Natus Medical
- HAIYING MEDICAL
- Camino Laboratories
- Jiangsu Yuyue Medical Technology Co., Ltd.
- Haiying Medical Technology Co., Ltd.
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential






 US: +1-(714)-364-8383
US: +1-(714)-364-8383






