MARKET OVERVIEW
The Indonesia In Vitro Diagnostics market, being a part of the overall medical diagnostics sector, will undergo dramatic change in the coming years not just based on technology, but on how it transforms medical interaction, access to healthcare, and interpretation of data. While the industry is commonly understood through the prism of laboratory development and technological advancements in detection systems, a closer look indicates that its true potential can be found in more fundamental, more human-level progress. It will no longer be limited to laboratory-based precision or hospital-grade equipment. Rather, it will model and be modeled upon networked healthcare systems, wellness perception shifts in culture, and the individualization of diagnostics on a small scale.
The Indonesia In Vitro Diagnostics market, in the future, will extend its reach beyond classical hospital environments. It will find its foothold in primary healthcare networks, rural outreach programs, and even DIY care in home environments. This path won't be merely about downsizing laboratory tests, but about intruding diagnostics within the everyday beat of human life. Picture a world where patients will look forward to illnesses not through abrupt symptoms, but through regular bio-signal monitoring prompted by humble household devices. That change will not only redefine the timeline within the clinical setting but will reshape the emotional and financial burden of delayed diagnosis.
Changes in demography and culture will also drive this market to be more accessible and responsive. The younger generations will insist on quicker, smarter, and more transparent diagnostic routes, while the elderly will need constant monitoring solutions that are less clinical and more reassuring. The Indonesia In Vitro Diagnostics market will thus have to evolve into a lifestyle companion merging scientific precision with psychological comfort. The test won't just be to identify a disease, but to explain it meaningfully, in empathetic and understanding languages that enable effective change.
The technological future also suggests the integration of diagnostics with AI, not merely for velocity but for interpretation infused with contextual insight. In coming applications, these systems will no longer simply provide results they will learn based on user history, environmental conditions, and genetic models. This will create a new diagnostic story, in which machines will not merely be tools, but guides that provide options, not data points. The Indonesia In Vitro Diagnostics market will have a greater impact on therapeutic decisions, lifestyle planning, and even mental health solutions by virtue of their accuracy and scope.
One must notice that the market will expand in scope but will also have increased accountability. Ethical norms, data rights, and consent systems will have to be as sound as the technology. It will no longer suffice to provide precision without responsibility. The next few years will require a diagnostics industry that fosters trust at all levels from the producer to the consumer through transparency, safety, and long-term contact.
In this broader perspective, the market will not merely serve medicine but will be a part of remapping society's perceptions of health, prevention, and personal agency. Its tale will be less of advancement and more of connection.
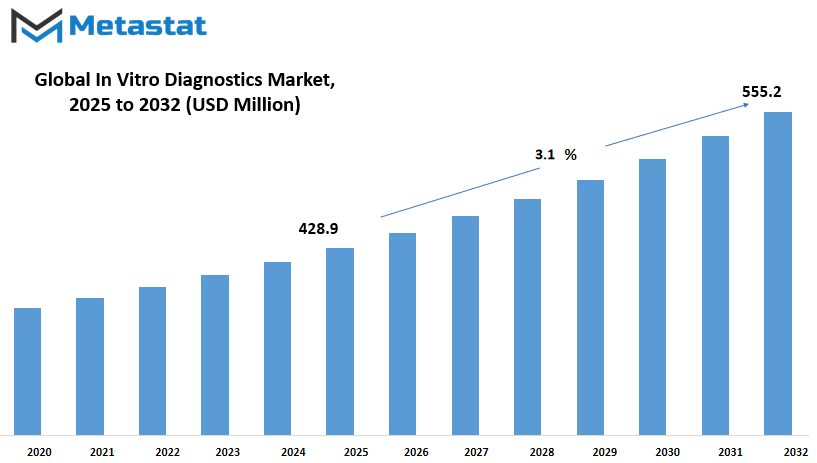
Indonesia In Vitro Diagnostics market is estimated to reach $555.2 Million by 2032; growing at a CAGR of 3.1% from 2025 to 2032.
GROWTH FACTORS
The Indonesia In Vitro Diagnostics market is revolutionizing the healthcare industry via enabling physicians to discover illnesses speedy and accurately. Among the most powerful drivers of this marketplace is the increasing incidence of infectious and continual diseases Indonesialy. With greater people laid low with illnesses consisting of diabetes, most cancers, or respiratory illnesses, the demand for precise and well timed diagnostic equipment will increase. Medical practitioners now rely on sophisticated technology which might be capable of offer specific outcomes inside shorter spans of time, improving remedy fees. This increasing reliance on diagnostics is complemented by means of some other key trend the growing application of personalised medicinal drug. As no longer all treatments are universally powerful, customized answers call for extra precise diagnostic information, and thereby gas demand for shrewd checking out device.
Though the fashion is on the upward push, the market for diagnostics has some issues to contend with. Among the biggest limitations is the economic burden of state-of-the-art diagnostic equipment. In poorer nations, clinics and hospitals are commonly now not in a role to purchase newer technologies, for this reason limiting patients' ability to get entry to timely and effective treatment. This leaves an opening in fitness first-class between advanced and growing international locations. Moreover, stringent law procedures complicate the process for companies to introduce new diagnostic gadget to the marketplace. The approval processes are time-ingesting and require large-scale scientific checking out, which slows product introductions and innovation at some point of the enterprise.
Despite these limitations, new opportunities are emerging that will redefine the course of the market. Point-of-care testing, for instance, is attracting notice for providing results outside of the clinical lab. From rural health clinics to ERs, these rapid tests enable quicker decision-making and are particularly helpful in outbreak situations when minutes count. Their simplicity and portability make them an asset in developed and developing health systems alike and enable more people to receive care in a timely fashion.
Another such promising factor is the growth of healthcare infrastructure in the emerging economies. Asian, African, and Latin American countries are developing hospitals, laboratories, and diagnostic centers. The growth brings diagnostic equipment within reach of more and more people, which means more demand for basic as well as sophisticated testing. As these economies further grow and develop, the demand for IVD technology will grow, which would provide companies with an opportunity to access new markets and expand their Indonesia presence.
Over the next few years, the Indonesia In Vitro Diagnostics market will continue to grow as healthcare models change and patient demands are more specific. Though cost and regulation are issues, pressure to move faster, be more accessible, and test more personally means that innovation will keep up. Medical demand, technological advances, and improvements in world infrastructure form a solid platform for the future of diagnostics.
MARKET SEGMENTATION
By End User
The Indonesia In Vitro Diagnostics market has continuously elevated because it plays a very critical role in disease identification and remedy choice-making. With the healthcare systems shifting toward preventive care and early prognosis, the want for powerful diagnostic products has emerge as ever greater important. In vitro diagnostics (IVD) is checks conducted on samples consisting of blood or tissue withdrawn from the human frame, and those tests resource within the detection of infections, tracking chronic illnesses, and measuring ordinary fitness. With increasing lifestyle situations and infectious outbreaks, the marketplace has attracted extra hobby from healthcare providers, policymakers, and investors.
Advances in medical technology and automation have advanced diagnostic checking out to be quicker, extra precise, and more convenient. Consequently, greater healthcare centers in diverse regions are using current diagnostic system to beautify patient consequences. The software of molecular diagnostics, immunoassays, and subsequent-generation sequencing has improved enormously. These advances are allowing clinicians to make extra informed decisions, mainly in essential conditions. The growing number of aged citizens, who commonly want consistent surveillance and common medical critiques, is likewise driving the demand for diagnostic offerings even better.
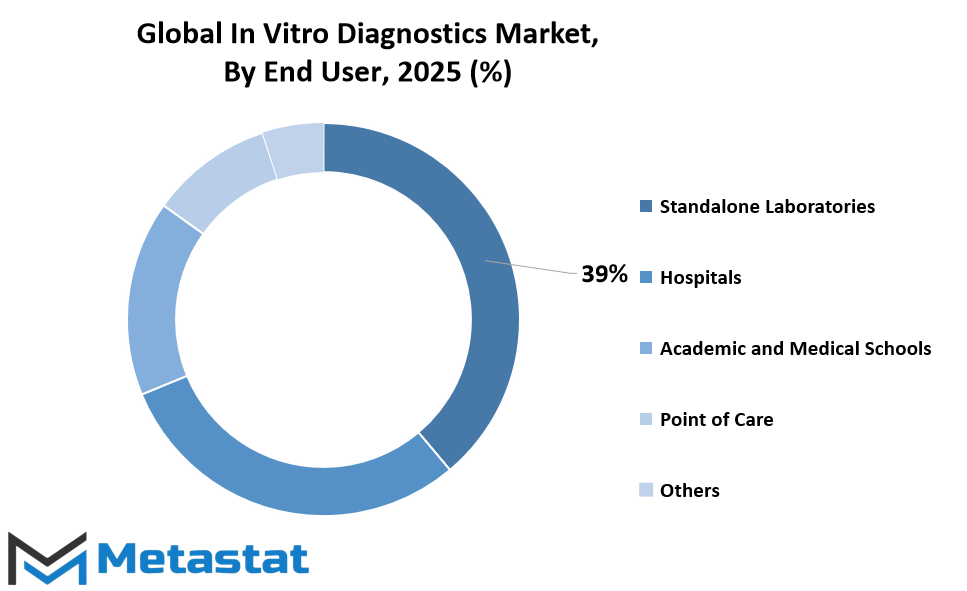
In vitro diagnostics are not needed only in hospitals or research institutes. The scope is increasing in different end-user environments. Independent laboratories, worth $166.7 million, remain an important contributor because of their wide range of testing options and effective delivery of services. Hospitals are also a significant segment with their centralized labs and the number of patients needing rapid diagnostic turnaround. Academic and medical schools contribute by way of research and education to ensure that innovation is perpetuated and new professionals are provided practical know-how. Point-of-care venues are becoming increasingly popular due to being able to provide rapid results without the need for elaborate lab equipment, particularly in rural and low-resource environments. Furthermore, the "others" category comprises diagnostic use in home care, clinics, and specialty centers, all contributing to the overall Indonesia In Vitro Diagnostics market value.
The Indonesia In Vitro Diagnostics market isn't always without troubles, but one of the foremost issues is the fee of advanced diagnostics in low-income regions. Limited infrastructure and insufficient trained personnel similarly prevent the implementation of recent gear in a few regions. In addition, adherence to regulatory requirements and records safety legislation remains the want for ongoing refocusing and retooling with the aid of carrier carriers and producers. Despite those challenges, the arena is promising because of its direct relationship with human fitness improvement and its pivotal function inside the scientific selection-making process.
In the destiny, the Indonesia In Vitro Diagnostics market will continue to transport closer to more handy and personalised solutions. As health attention rises and era becomes greater available, greater human beings will pursue recurring diagnostic screening no longer only for ailment, however for prevention. This better awareness and the increased use of diagnostics with digital health equipment will revolutionize the future of hospital treatment, permitting improved, faster, and greater informed selections at all ranges of the healthcare system.
By Product and Services
The Indonesia In Vitro Diagnostics market will persist in defining the manner in which medical professionals identify and track conditions Indonesialy. As novel health concerns surface and patient demands increase, this market will play a pivotal role in facilitating correct and timely diagnosis. Chronic diseases' continuous growth, as well as an increased emphasis on personalized medicine, equates to continuous growth in the demand for in vitro diagnostic technologies in the years ahead.
The largest share of the market exists in reagents and kits, which include the chemical reagents and check assemblies to carry out positive assays. These varieties of merchandise are the muse of diagnostic protocols, supporting everything from trendy blood work to superior molecular trying out. As labs seek to reduce turnaround time and growth sensitivity, those reagents and kits might be the recipient of investment by way of companies to beautify balance, shelf existence, and user-friendliness.
Instruments are some other critical segment, overlaying analyzers, immunoassay platforms, and molecular test machines. The gadgets are meant to automate and standardize operations, minimizing the capability for human mistakes and enhancing throughput. As labs are searching for to manner greater samples in greater extent with extra performance, demand will remain robust for compact, high-performance contraptions, inflicting producers to assume in phrases of pace, connectivity, and ease of protection.
Software answers end up ever greater vital as diagnostic information increases in amount and complexity. Laboratory records management systems, statistics‑evaluation software, and connectivity systems help clinicians and technicians with workflow management, end result interpretation, and secure sharing of consequences. As diagnostic centers and hospitals emerge as networked, software will fill the distance between uninterpreted facts and informed selection‑making, imparting dashboards, predictive evaluation, and remote monitoring.
In addition to products, services fill out the picture with installation, calibration, training, and technical support. These services will enable laboratories to get the most out of their investment and deliver quality consistently over the years. As the IVD market goes Indonesia, service networks will extend to new geographies with faster response times and localized knowledge. Working in concert, reagents and kits, instruments, software, and services will propel the Indonesia In Vitro Diagnostics market into its next growth cycle.
By Technique
The Indonesia In Vitro Diagnostics market is constantly increasing as healthcare structures everywhere in the globe make faster and more green way of ailment detection a concern. These assessments are performed outside the body by way of utilising samples along with blood, saliva, or tissue. They are critical in detecting infections, tracking health popularity, and informing treatment regimens. As people end up more and more knowledgeable about preventive health and early disorder detection, the demand for effective diagnostic device is becoming regularly sizeable.
Of all the techniques, molecular diagnostics has obtained a whole lot of emphasis. It is used to as it should be detect genetic material and is specially carried out in diagnosing viruses and bacteria, even before the onset of symptoms. The approach become pivotal at some point of the COVID-19 pandemic and remains useful whilst dealing with chronic diseases along with most cancers and inherited conditions. Its capacity to provide high-accuracy results quick has made it an accommodated approach in each medical and research environments.
Tissue diagnostics is another significant segment. This method emphasizes testing tissue samples, particularly to identify cancer and other cellular abnormalities. It is widely applied in hospitals and diagnostic labs to aid pathologists in giving accurate diagnoses. With the prevalence of cancer developing everywhere in the world, this phase for advanced tissue-based assessments can even boom, so this phase will remain a regular contributor to the general market.
Some of the critical techniques involve medical chemistry, that's used to quantify chemical substances in frame fluids to gauge organ characteristic; immunodiagnostics, which employs antibodies to diagnose infectious sicknesses like HIV and hepatitis; and hematology, which analyzes blood issues like anemia and leukemia. Each of those techniques has a particular role to play in improving affected person care and presenting early remedy. Other technology are nonetheless on the rise, offering new manner of trying out and tracking fitness higher.
With improving technology and reducing prices, more individuals will have access to these diagnostic techniques, particularly in the developing world. With an increase in older populations and the prevalence of chronic illness, the Indonesia In Vitro Diagnostics market shall grow due to innovation and an increase in the demand for effective healthcare solutions.
By Application
The Indonesia In Vitro Diagnostics market keeps expanding as medical science makes diagnostics faster, more precise, and more convenient. Fundamentally, in vitro diagnostics is all approximately testing out of doors the frame, commonly a laboratory, to locate illnesses or tune health states. These tests have grow to be vital gear of cutting-edge healthcare, helping physicians to make decisions with improved self assurance. What become once identified in days or weeks is now found inside hours, taking into account well timed treatment and stepped forward fitness outcomes for patients. This constant advancement in trying out has created increasing call for among numerous health situations and geographies.
Among the huge regions where those diagnostics are gambling an essential function is in identifying infectious sicknesses. In the face of world uptick in viral and bacterial infections, starting from the seasonal flu to greater risky ones which includes COVID-19 or tuberculosis, speedy and trustworthy diagnostic gadgets are important. The ability to detect an infection in the early levels controls its unfold and will increase recovery prospects. Increased availability of such checks in plenty of the developing world is now last lengthy-status health transport gaps. Infection checking out is most probable to maintain as a pinnacle market driving force and innovation force.
The detection of cancer is every other good sized use of in vitro diagnostics. Early prognosis can be the difference among powerful remedy and no longer. Using those exams, physicians are able to locate cancer markers in blood or tissue samples, permitting them to treat the disorder earlier than it becomes greater tough to deal with. This approach is not exclusive to most cancers. Cardiac conditions, one of the biggest killers worldwide, also gain from in vitro diagnostics. By detecting risks like high cholesterol or enzyme levels that may lead to heart attacks, these tests can warn patients and physicians prior to a severe health incident.
Immune system-related, kidney, and digestive disorders are also being treated with the assistance of in vitro diagnostics. Be it autoimmune diseases, kidney infections, or gastrointestinal problems, specific testing identifies the problem and allows physicians to recommend improved remedies. Each one of these uses provides new opportunities for research, development, and improved care. With more individuals Indonesialy being provided with improved healthcare, the need for such diagnostics will automatically increase.
Finally, there is increased recognition of the value of ongoing health surveillance, which helps spur the market's increase in lesser-documented or undocumented disease groups. In vitro diagnostics are not only for patients who already know they have something serious these are prevention tools as well. From routine blood work to individualized medicine, this industry is transforming what we consider healthcare. Moving forward, it will continue to shape both patient experience and medical practice in ways that are practical, personal, and life-changing.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$428.9 million |
|
Market Size by 2032 |
$555.2 Million |
|
Growth Rate from 2025 to 2032 |
3.1% |
|
Base Year |
2025 |
|
Regions Covered |
North America, Europe, Asia-Pacific Green, South America, Middle East & Africa |
REGIONAL ANALYSIS
Regional trends and healthcare agendas drive the Indonesia In Vitro Diagnostics market, which varies considerably by geographies. The United States dominates the North American region with an established healthcare system, swift technology uptake, and tremendous demand for personalized diagnostics. Canada and Mexico also contribute to the market, albeit on a relatively lower scale. Major diagnostic firms and continuous pressure to diagnose diseases early have transformed North America into a mature and developing market for in vitro diagnostic solutions. Additionally, government incentives and population awareness continue to increase demand for precise and prompt diagnostic testing.
Europe is closely following suit, led by Germany, the UK, France, and Italy. These countries have robust healthcare systems and high levels of research and development spending. Demand for in vitro diagnostics in these countries is linked to the aging of the population, increasing rates of chronic diseases, and expanding focus on preventive medicine. Each of the countries has its own regulatory and reimbursement structures, which are supportive or hindering of new technologies. In spite of this, Europe is steady in its growth, with the Rest of Europe contributing equally through emerging markets that are starting to improve their healthcare provision.
Asia-Pacific, where densely populated nations such as India and China are located, is highly promising because of rising healthcare expenditure and awareness. With their economies growing further, so does their capacity to spend on advanced medical solutions. Japan and South Korea, being technologically advanced themselves, possess strong diagnostic services that play a valuable role in the regional market. What is most interesting about Asia-Pacific is the mix of well-developed markets and accelerating developing regions that, combined, generate a very interesting environment full of opportunity for expansion and innovation in the diagnostics market.
In South America, Brazil and Argentina are the main contributors. The two nations are enhancing access to healthcare and investing in healthcare technologies. Nevertheless, issues such as economic instability and maldistribution of healthcare resources remain to impact progress. Despite this, there is evidence of growth, particularly as the public and private health sectors start to prioritize early diagnostics in order to better manage disease burdens. The Rest of South America, though smaller in terms of market size, is catching up slowly as initiatives towards modernizing healthcare infrastructure begin to take form.
The Middle East & Africa region is also emerging as a focal point, with GCC nations such as Saudi Arabia and the UAE investing significantly in healthcare infrastructure. Egypt and South Africa add their share by increasing demand for diagnostic equipment, especially in fighting infectious and chronic diseases. Restricted access and unequal distribution of resources are still issues in some of the nations in this market, but the demand for good diagnostics drives the market ahead. With an increasing number of countries in this region making healthcare improvements a top priority, demand for in vitro diagnostics will continue to rise.
COMPETITIVE PLAYERS
The Indonesia In Vitro Diagnostics market continues to pick up steam as the need for accurate, efficient, and affordable medical testing increases throughout healthcare systems. With growing knowledge of the importance of early detection of disease and tailored treatment, diagnostic solutions are not an option anymore they're necessities. Governments, private health providers, and research organizations are putting more focus on enhancing diagnostics to enable public health and lower long-term costs of disease. As lifestyle diseases and infectious illnesses affect populations, the demand for reliable diagnostic equipment will increasingly be felt in the developed and developing world.
Indonesia has become a major force in this sector with an increasing number of companies that are actively involved in innovation and supply. Major players in the sector are PT Pakar Biomedika Indonesia and PT Biocare Sejahtera, who engage in manufacturing and selling diagnostic solutions that are locally responsive. Biofarma Group and PT Elvinco Med Tech have also made a solid presence, contributing to advancing technologies to conform to international standards but made available in regional healthcare environments. They have partnered with others like Fapon Biotech Indonesia and PT. Healthy Indonesian Genbody, which provide a blend of technical competency and market sensitivity.
This growth of the Indonesia In Vitro Diagnostics market is fostered by initiatives from companies such as Interskala Medika, Jayamas Medica Industry, PT. KalGen DNA, and PT Konimex Diagnostic. Their activity runs the gamut from genetic testing to quick point-of-care diagnostics with the aim to enhance turnaround times and widen availability. PT Merah Putih Manufaktura and PT Sedoyo Sami Sehat are also putting in their share towards manufacturing capacities in Indonesia, which means less reliance on imported kits and more local innovation. Meanwhile, Sentra Medika and PT Tempo Scan Pacific Tbk are contributing to both distribution and public education, making sure that diagnostic equipment reaches the clinics and hospitals in various regions.
Virtue Diagnostics Indonesia is among the recent but prominent contributors, reflecting the sector's acceptance of new players and technologies. With growing competition comes greater quality and affordability of diagnostic services. This movement lends support to expanded access, particularly for under-resourced health centers that have long faced delays in diagnostics. Multiple stakeholders manufacturers, laboratories, and health providers are collaborating to build a more responsive and effective diagnostic infrastructure.
As the international focus continues to be on readiness and quick response in health, the In Vitro Diagnostics market will keep acting as an important backbone for health decision-making and treatment planning. Businesses in this area are not just building their technology scope but are also evolving towards regulatory changes and shifting health priorities. The trend toward faster, more accurate, and affordable diagnostics is a positive one for the world's healthcare, and Indonesia's expanding industry is poised to play a significant role in advancing that trend.
Indonesia In Vitro Diagnostics Market Key Segments:
By End User
- Standalone Laboratories
- Hospitals
- Academic and Medical Schools
- Point of Care
- Others
By Product and Services
- Reagents and Kits
- Instruments
- Software and Services
By Technique
- Molecular Diagnostics
- Tissue Diagnostics
- Clinical Chemistry
- Immunodiagnostics
- Hematology
- Others
By Application
- Infectious Diseases
- Cancer
- Cardiac Diseases
- Immune System Disorders
- Nephrological Diseases
- Gastrointestinal Diseases
- Others
Key Indonesia In Vitro Diagnostics Industry Players
- PT Pakar Biomedika Indonesia
- PT Biocare Sejahtera
- Biofarma Group
- PT Elvinco Med Tech
- Fapon Biotech Indonesia
- PT. Healthy Indonesian Genbody
- Interskala Medika
- Jayamas Medica Industry
- PT. KalGen DNA
- PT. Konimex diagnostic
- PT Merah Putih Manufaktura
- PT Sedoyo Sami Sehat
- Sentra Medika
- PT Tempo Scan Pacific Tbk
- Virtue Diagnostics Indonesia
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential





 US: +1 3023308252
US: +1 3023308252






