MARKET OVERVIEW
The global medical device validation and verification market is a niche area in the medical technology market that will continue to gather interest due to its contribution toward ensuring product safety and accuracy. The market will form the cornerstone of device quality assurance in the areas of diagnosis, monitoring, and treatment. With increasing regulatory requirements from various nations becoming more defined and harmonized, the need for in-depth validation and verification will keep increasing.
Validation and verification in this industry will not be mere compliance checkboxes. Rather, they will become strategic functions built into product development processes. Each new medical device brought to market will see thorough testing to guarantee that it performs as designed under anticipated conditions. Verification will be aimed at ensuring every component or system conforms to its design specifications, whereas validation will prove that the end product is meeting user requirements in practical situations. These processes will not be done in isolation but together with engineering, design, clinical, and regulatory groups.
The form of this market will go beyond standardized protocols and tests. It will require more in-depth commitment to process optimization, method development, and iterative testing. The stakeholders will be global regulatory agencies, contract research organizations, third-party laboratories, and in-house quality control staffs. Their collective work will define the measures against which devices are evaluated, constantly raising the bar for what is considered acceptable performance.
The global medical device validation and verification market will be guided by innovation and scrutiny. New technologies, including connected devices and AI-driven systems, introduce new levels of complexity and call for novel testing paradigms. Conventional approaches will no longer be adequate for devices that dynamically interact with users and external systems. The transition will test validation teams to develop more fluid models that reflect the contexts where devices will be used.
Different geographical regions will contribute distinct perspectives to this market. For example, Europe will continue to emphasize stringent pre-market evaluation under the MDR, while the United States will focus on risk-based pathways guided by the FDA. Meanwhile, countries in Asia-Pacific will adapt these global approaches while accounting for localized healthcare priorities. The result will be a dynamic market influenced by both harmonized policies and regional interpretations.
Service providers in this field will customize their services to particular device classes, from Class I disposable devices to high-risk implantable systems. Their services will range from feasibility studies early on to post-markets surveillance, producing an ongoing feedback loop for performance refinement. Software evolution of devices will introduce an added dimension of complexity, particularly as validation activities increase coverage to firmware updates as well as cybersecurity considerations.
Forward-looking, the global medical device validation and verification market will have a significant impact on forming device safety and reliability. Since healthcare becomes increasingly dependent on technology, the standards used to test and approve these instruments will have to keep up. Through this, this market will get to determine the fate of clinical performance, user safety, and regulatory confidence among worldwide healthcare systems.
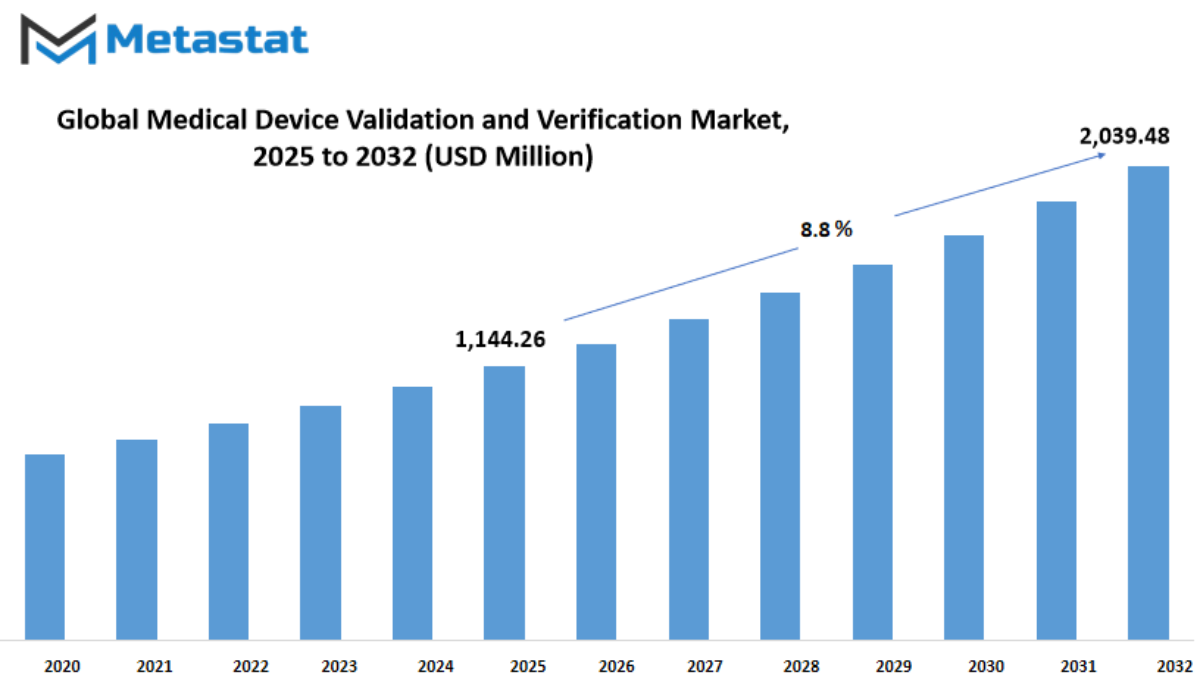
Global medical device validation and verification market is estimated to reach $ 2,039.48 Million by 2032; growing at a CAGR of 8.8% from 2025 to 2032.
GROWTH FACTORS
The global medical device validation and verification market is expected to grow steadily in the years ahead, supported by several key factors that shape its direction. One of the strongest driving forces behind this growth is the rising focus on patient safety and the need to meet strict regulatory standards. Medical devices must prove their safety and performance through proper validation and verification, and this requirement is only expected to get more serious in the future. As more advanced technologies are introduced into healthcare, the demand for thorough testing will rise. New devices, especially those that involve digital health solutions or artificial intelligence, will require careful examination before they can be used widely. This growing complexity in product design will naturally push companies to invest more in ensuring their devices meet all safety and performance expectations.
Another important factor is the aging global population. As people live longer, the need for medical devices increases, especially for chronic conditions. Devices used for diagnosis, monitoring, and treatment must be accurate and dependable, which places even greater importance on validation and verification processes. Healthcare systems in both developed and developing regions will continue to adopt more devices, which means more rigorous quality checks will be necessary. This trend is likely to strengthen the market's growth for years to come.
However, some factors might slow down the progress of the global medical device validation and verification market. One of these is the high cost involved in conducting proper testing. Smaller companies, in particular, may struggle to afford these processes, which could delay product launches or limit innovation. In addition, varying regulations across different regions can create confusion and slow down approvals, especially for companies aiming to enter multiple markets at once.
On the other hand, the future holds promising opportunities. Growing digitalization in healthcare, the use of simulation and modeling tools, and the integration of advanced software into medical devices are all creating new ways to perform validation and verification more efficiently. These developments can reduce time and cost while improving accuracy. In the coming years, the use of virtual testing environments and artificial intelligence could open up faster and safer ways to test devices before they reach patients. This shift could make the process more accessible, especially for companies trying to scale quickly.
Overall, with the ongoing innovation in medical technology and rising expectations around patient safety, the global medical device validation and verification market will likely continue expanding, driven by the need for dependable and high-quality medical solutions.
MARKET SEGMENTATION
By Validation Type
The global medical device validation and verification market is expected to grow steadily as technology continues to influence the way healthcare is delivered. As medical devices become more advanced and play a larger role in diagnosing, monitoring, and treating patients, the need to ensure their safety, reliability, and performance will increase. Validation and verification are essential steps in making sure these devices work as intended and meet all regulatory standards. Over the coming years, this focus on thorough testing and approval processes will only become more important as the market expands.
By validation type, the global medical device validation and verification market can be divided into several parts, each with its own purpose. Design validation checks whether a medical device will meet the needs of users when it is finally made. This is done by testing early models or prototypes under real or simulated conditions. In the future, new tools such as virtual simulations and 3D modeling might make design validation more accurate and faster. Process validation, on the other hand, ensures that the steps used to produce a device will always result in a quality product. With smart manufacturing and AI-based monitoring becoming more common, this type of validation will likely become more automated and data-driven.
Product verification makes sure that a device was made according to the approved design and that each part meets required standards. As production methods improve, this step may involve more real-time monitoring and digital records to spot issues early. Clinical validation is also very important, especially for devices used in diagnosis or treatment. It checks whether the device gives results that are correct and helpful for medical decisions. In the years ahead, more attention will be given to clinical trials and studies that reflect different patient needs and health conditions. Other types of validation may include software validation or package testing, which will also gain more focus as digital health and safety measures become a bigger part of device use.
Overall, the global medical device validation and verification market will keep growing as medical technology advances and health regulations become more detailed. As companies aim to bring new products to market faster without risking quality or safety, validation and verification will play a larger role in the development cycle. Future trends such as automation, AI, and digital tracking will shape how these processes are handled, making them more efficient and reliable. The demand for trusted devices will make this market a key part of global healthcare progress.
By Category
The global medical device validation and verification market is expected to grow steadily as healthcare systems around the world continue to improve and adapt to new technologies. This market will become more important as medical devices become more complex, requiring careful checks before they can be used safely. As technology continues to change how healthcare is delivered, there will be more demand for strict testing methods that make sure medical devices work the way they should and do not pose any risk to patients or healthcare professionals.
The global medical device validation and verification market is divided into several categories, including In-Vitro Diagnostics, Surgical Instruments, Implantable Devices, and Monitoring Equipment. Each of these categories has its own set of challenges and standards that need to be met. For example, In-Vitro Diagnostics must show accurate results every time, while Surgical Instruments need to be tested for both safety and performance. Implantable Devices go through some of the strictest testing processes because they stay inside the body for long periods. Monitoring Equipment must prove it can give real-time, reliable data to help doctors make quick and correct decisions. As we move forward, these devices will become more advanced, meaning the testing process will need to grow with them.
Looking ahead, the global medical device validation and verification market will see growing attention from both manufacturers and regulators. Devices will be expected to meet higher standards, especially with the growing use of artificial intelligence, sensors, and connected health systems. These features will need to be tested in ways that were not required before, which will add new steps to the validation and verification process. It will not be enough for a device to work; it will need to prove that it works in many different situations, under different conditions, and with different users.
As the demand for personalized medicine and home healthcare rises, medical devices will need to be tested for use outside of hospitals. This shift will push companies to develop more flexible and user-friendly testing methods. The global medical device validation and verification market will be a key part of making sure that these changes lead to safe and effective healthcare for everyone. Over time, these efforts will help build trust in medical technology, support faster approvals, and lead to better health outcomes around the world.
By Service Type
The global medical device validation and verification market is set to grow steadily as technology continues to shape healthcare in the years ahead. These services play a major role in ensuring that medical devices work properly and meet required safety standards before reaching hospitals, clinics, or patients. By focusing on service types, the market is broken down into four areas: Consulting Services, Testing Services, Certification Services, and Audit Services. Each of these is expected to become even more important as regulations grow stricter and devices become more advanced.
Consulting Services are likely to gain more attention in the future. As more complex devices are being created, manufacturers will need expert guidance to understand new rules, avoid delays, and ensure their devices are approved for use. This will push demand for professionals who can offer advice tailored to both global and regional regulations. These services will also help companies avoid mistakes early in the process, which could save time and costs later.
Testing Services are another essential part of this market. In the future, with more wearable devices, smart implants, and robotic tools being developed, testing will need to be even more detailed. It will not just be about safety but also about how well these tools perform over time and under different conditions. This means the need for real-world simulations and reliable testing methods will continue to grow.
Certification Services are expected to remain a key step in getting any device approved. With global rules constantly changing, having a clear and official stamp of approval will still matter. It will give buyers, users, and regulators confidence in a product. Certification will also help companies enter new markets faster, especially as healthcare becomes more connected and patient-focused across countries.
Audit Services will continue to help companies stay on track. In the future, audits will not just focus on paperwork or checking boxes. They will be used more as tools to improve processes, catch issues early, and adjust to new requirements quickly. This could also include remote audits using digital tools, which might become common as global teams work together across time zones.
Overall, the global medical device validation and verification market will be shaped by new technology, changing regulations, and a stronger focus on safety. These service types will play a major role in helping companies bring reliable and approved devices to the people who need them, both now and in the future.
By End-Use
The global medical device validation and verification market is gradually shaping the future of healthcare, playing a crucial role in ensuring the safety and effectiveness of medical equipment. As healthcare systems across the world expand, the need to check and confirm that medical devices work as expected becomes more important than ever. This process isn't just a technical requirement it will help build trust among healthcare professionals and patients alike. Looking ahead, this market is likely to grow steadily as the demand for high-quality healthcare rises, along with the growing complexity of medical devices.
Hospitals are one of the main users of medical device validation and verification services. With the increasing number of patients and advanced tools being used in modern hospitals, there will be a stronger push to make sure all devices function properly before they are used in real-life situations. From diagnostic tools to surgical machines, every piece of equipment must pass strict checks to avoid any risk to patient safety. Hospitals will continue to rely on this process to reduce errors and improve treatment outcomes, especially as newer and smarter technologies are introduced into routine care.
Clinics, though smaller in size, are also important players in this market. They often serve as the first point of care for many people and use a wide range of devices to examine and treat patients. These clinics will increasingly depend on validation and verification to make sure the tools they use are reliable. This is especially true as more outpatient procedures are being done in clinic settings, which means more pressure to use trusted equipment.
Research laboratories also contribute to the growth of the global medical device validation and verification market. These labs play a key part in developing new medical devices, and every prototype must go through thorough testing before it can move forward. In the future, more labs will adopt advanced testing methods to speed up development without cutting corners on safety. This trend will push for stricter and more refined validation practices.
Pharmaceutical companies, although mostly focused on drugs, are using more devices in drug delivery and diagnostic tools. As these devices become a bigger part of treatment plans, companies will rely heavily on strong validation and verification practices. Looking ahead, their role in this market will likely grow as they explore ways to combine medicine and technology for better results.
As these different users continue to rely on dependable devices, the market will keep expanding and improving the future of healthcare.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$1,144.26 million |
|
Market Size by 2032 |
$ 2,039.48 Million |
|
Growth Rate from 2025 to 2032 |
8.8% |
|
Base Year |
2024 |
|
Regions Covered |
North America, Europe, Asia-Pacific Green, South America, Middle East & Africa |
REGIONAL ANALYSIS
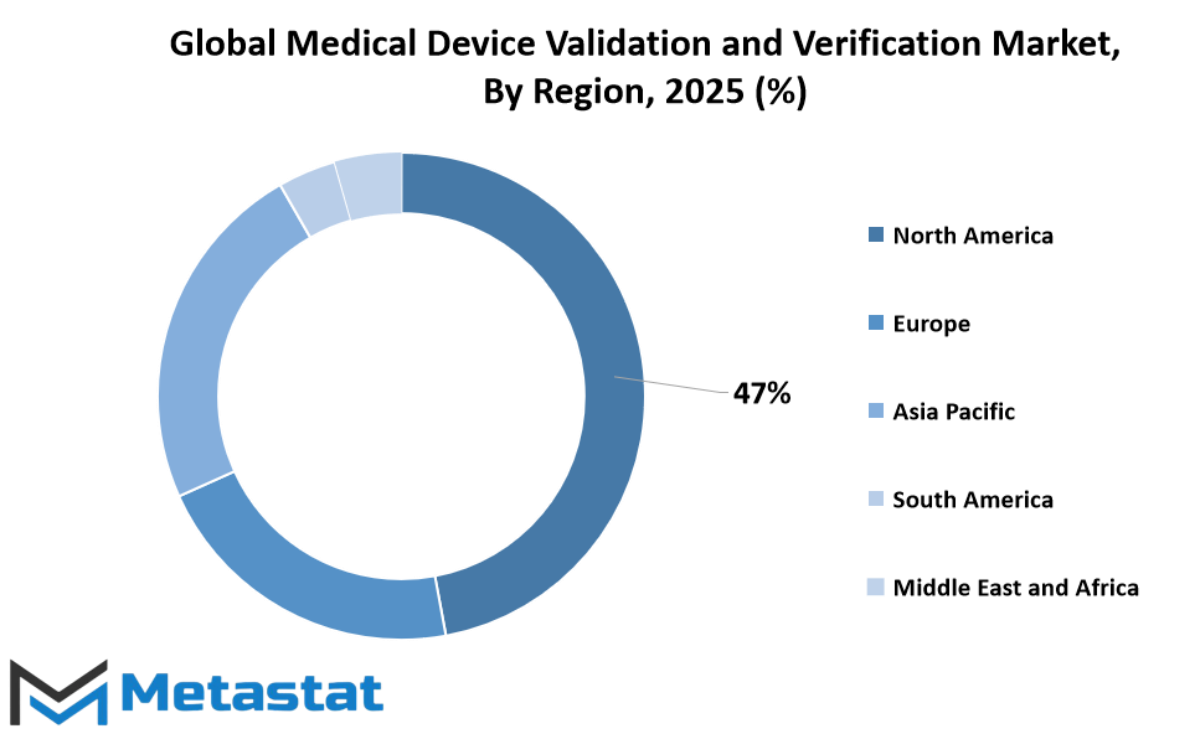
The global medical device validation and verification market is gaining attention as healthcare systems around the world continue to evolve and place greater emphasis on safety, reliability, and innovation. As devices become more advanced, ensuring they meet strict quality standards is no longer optional it’s essential. This demand for dependable and consistent devices is driving growth across different regions, each playing a unique role in shaping the market’s future.
In North America, countries like the United States, Canada, and Mexico are leading the charge. The U.S., with its strong regulatory frameworks and well-established healthcare industry, is expected to continue investing in technologies that push for better compliance and faster testing procedures. Canada and Mexico are also improving their medical infrastructure, and that will increase the need for precise validation and verification processes.
Europe follows closely, with major contributors such as the UK, Germany, France, and Italy focusing on patient safety and strict adherence to regulatory guidelines. This region values product transparency and consistent quality, which creates a strong environment for the growth of the global medical device validation and verification market. Countries in this area will likely keep adapting to changes in technology and expand their investments in systems that ensure devices are not just effective but also safe for long-term use.
In Asia-Pacific, the picture looks very promising. India, China, Japan, and South Korea are becoming hubs for both manufacturing and development of medical devices. These countries have seen fast growth in healthcare demands, and governments are responding by strengthening safety standards. As healthcare access improves across the region, so will the focus on validating and verifying medical devices before they reach hospitals and clinics. This forward-thinking approach will help Asia-Pacific become a powerful force in the market.
South America, led by Brazil and Argentina, is also building momentum. Efforts are being made to improve healthcare systems, which will naturally increase the need for strong validation procedures. As new policies and reforms roll out, they are expected to open more doors for the expansion of the global medical device validation and verification market in this part of the world.
In the Middle East & Africa, including areas such as the GCC Countries, Egypt, and South Africa, healthcare modernization is moving steadily. Investment in hospitals and medical technologies is creating a growing need for trusted verification systems. As infrastructure continues to improve, this region will also play a bigger role in shaping the future of this global market.
COMPETITIVE PLAYERS
The global medical device validation and verification market is expected to witness steady growth in the years to come. As technology continues to influence the development of medical devices, the need for accurate testing, validation, and verification will become even more important. These processes are crucial in ensuring that devices are safe, effective, and meet the required regulatory standards before reaching patients. As healthcare systems around the world shift their focus toward precision, safety, and performance, this market will continue to play a central role in shaping the future of medical technology.
Companies working in this space are already showing signs of adapting to the changing needs of the industry. Key players such as TÜV SÜD, SGS SA, Intertek Group plc, and Eurofins Scientific are pushing forward with improved testing protocols that not only meet current guidelines but also prepare for stricter standards likely to appear in the future. These companies, along with UL Solutions and Element Materials Technology, are investing in advanced tools and software to reduce errors and ensure quicker approval times for devices. The rising complexity of modern medical equipment demands a more refined approach to how they are tested, and these businesses are stepping up to that challenge.
In the future, we will likely see more collaboration between regulatory bodies and testing organizations to set global benchmarks. This will help streamline the process for manufacturers and increase patient safety. Companies like DNV, NAMSA, and Charles River Laboratories are expected to continue focusing on specialized services tailored to new device classes, particularly those that use digital or AI-powered features. As these types of devices enter the market, there will be a greater need for validation processes that not only confirm the physical integrity of the product but also evaluate how software elements behave under real-world conditions.
Medistri SA, Europlaz Technologies, and Pace Analytical are also playing their part by offering services that focus on quality control and testing under different environmental factors. These efforts help ensure that devices perform well not just in ideal conditions, but also when exposed to real-life use. Moving forward, this will become even more important as the global market expands into regions with different climates, usage patterns, and patient demographics.
As innovation drives the development of new devices, the global medical device validation and verification market will keep evolving. The companies involved are not only meeting today's standards but also preparing for what comes next, helping ensure that future medical technologies are safe, reliable, and ready for global use.
Medical Device Validation and Verification Market Key Segments:
By Validation Type
- Design Validation
- Process Validation
- Product Verification
- Clinical Validation
- Other
By Category
- In-Vitro Diagnostics
- Surgical Instruments
- Implantable Devices
- Monitoring Equipment
By Service Type
- Consulting Services
- Testing Services
- Certification Services
- Audit Services
By End-Use
- Hospitals
- Clinics
- Research Laboratories
- Pharmaceutical Companies
Key Global Medical Device Validation and Verification Industry Players
- TÜV SÜD
- SGS SA
- Intertek Group plc
- Eurofins Scientific
- UL Solutions
- Element Materials Technology
- DNV (Det Norske Veritas)
- Element Materials Technology
- NAMSA (North American Science Associates)
- Pace Analytical
- Charles River Laboratories
- Medistri SA
- Europlaz Technologies
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential





 US: +1 3023308252
US: +1 3023308252






