
Aug 07, 2025
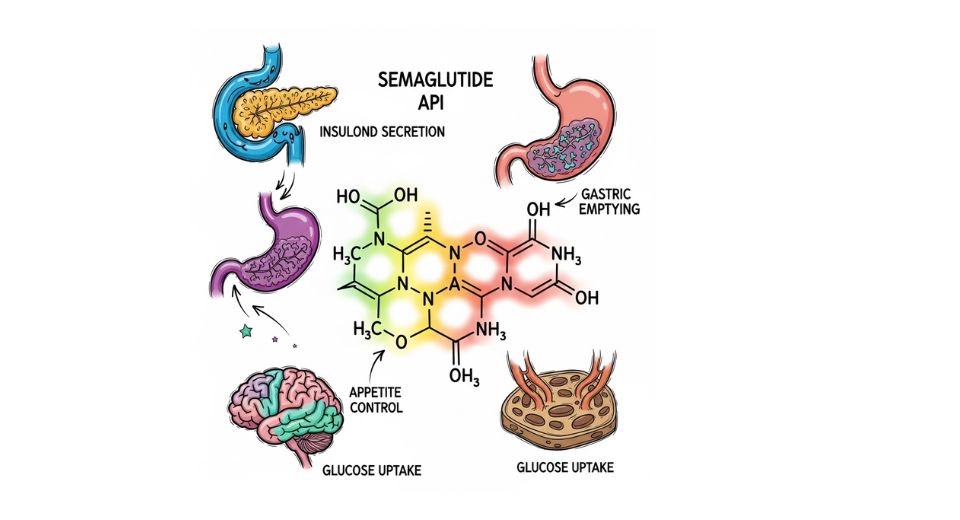
The just-released report from Metastat Insight provides fresh insights into the Global Semaglutide API Market, presenting a thorough examination of a sector that remains in the spotlight among pharmaceutical developers, health strategists, and investors globally. The report examines the business and scientific aspects of this singular environment, seizing both the present situation and the overall climate influencing its trajectory. This market, defined by changing healthcare priorities and regulatory environments, is not simply a category of pharmaceutical production it is a complex and multifaceted environment characterized by the modest variations in strategy, investment, and innovation.
One of the most salient features of this market is the fine balance of demand and availability. With public health interventions focusing more on managing chronic diseases, the function of APIs like semaglutide comes to bear even more significance. Processes by which these substances arrive in the final product entail a complex series of value-added operations, not only in chemical synthesis but also conformity, scalability, and distribution. Firms that engage in this process need to be highly specialized, not just to achieve regulatory levels but also to compete within an industry characterized by precision and high stakes.
There is a pattern of regional specialization throughout a number of regions reflected in this market. It is not uncommon to discover that one geography specializes in one segment of the production process, with another becoming identified with distribution or research. These technical centers engage with each other over borders, creating a more collaborative and less siloed global architecture. In this scenario, the Global Semaglutide API Market is less a matter of isolated progress and more one of collaborative progress determined by multiple regulatory environments as well as different commercial motivations.
What makes this market differ from others within the larger pharmaceutical space is its reliance on technical prowess and process consistency. Semaglutide, with its particular structural and functional features, requires consistency of manufacture that only a select number of companies can achieve at volume. This, in its turn, restricts the number of potential competitors and presents barriers to market entry that serve both as a shield and a filter. Only the firms with the ability to match scientific rigor with logistical accuracy achieve long-term success. This dynamic generates a high barrier for new entrants, but also creates a closely regulated competitive landscape where innovation is developed with well-defined boundaries.
Supply chains in this segment are meticulously designed, sometimes with extended planning horizons and stringent quality checks. These chains have been tested in recent years by evolving geopolitical realities and public health crises, which exposed weaknesses that are being addressed with new sense of urgency. The ability to react to such pressures has been a distinguishing feature of those businesses that still retain market share. However, far from being a retreat into risk aversion, many stakeholders have welcomed this as a moment that requires more transparency, improved planning, and spending on supply resilience.
From a commercial perspective, the pricing models and partnership arrangements in the Global Semaglutide API Market also have interesting contours. Licensing arrangements, co-manufacturing joint ventures, and technology transfers increasingly define the production and delivery of semaglutide APIs. Their complexity echoes the wider significance of trust, experience, and track record in this business. Though these deals tend to be done out of the spotlight, their influence on market organization and availability of output is both profound and extensive.
At the same time, innovation is occurring on two fronts: process improvement and diversity of applications. On the one hand, producers are investing in more effective synthesis routes to minimize waste and expense. On the other hand, research organizations and private companies are looking into the capabilities of semaglutide APIs in therapeutic applications that exceed existing uses. These studies will not always yield immediate results, but they remind us that scientific investigation is still a part of this market's constant evolution. The dynamic interaction between commercial need and scientific breakthrough still pushes the quiet evolution of direction in development forward, even when it seems on the surface that little is being accomplished.
A second overarching theme in the history of this market is regulation. Regulators across jurisdictions have different expectations, but there is nevertheless a consistent move toward harmonization. That has the effect of facilitating cross-border trade and providing fresh opportunities for businesses that can fairly easily move through multilayered regimes of compliance. How well those companies can learn to adjust to changing requirements without compromising quality is now a metric of long-term sustainability in this industry.
As the Metastat Insight report on the Global Semaglutide API Market identifies, this market is an intricate, dynamic landscape defined by cooperation, technical specificity, and strategic vision. Far from being a monolithic, static entity, the market itself is defined by several interconnecting streams of influence each lending its own character to a structure as responsive as it is robust. It manifests a unique combination of scientific rigor and commercialism, underpinned by collaborations that balance both innovation and continuity. The report is more than a snapshot, and instead gathers the dynamic conversation between progress and limitation, ambition and pragmatism.
Drop us an email at:
Call us on:
+1 214 613 5758
+91 73850 57479