
Nov 05, 2025
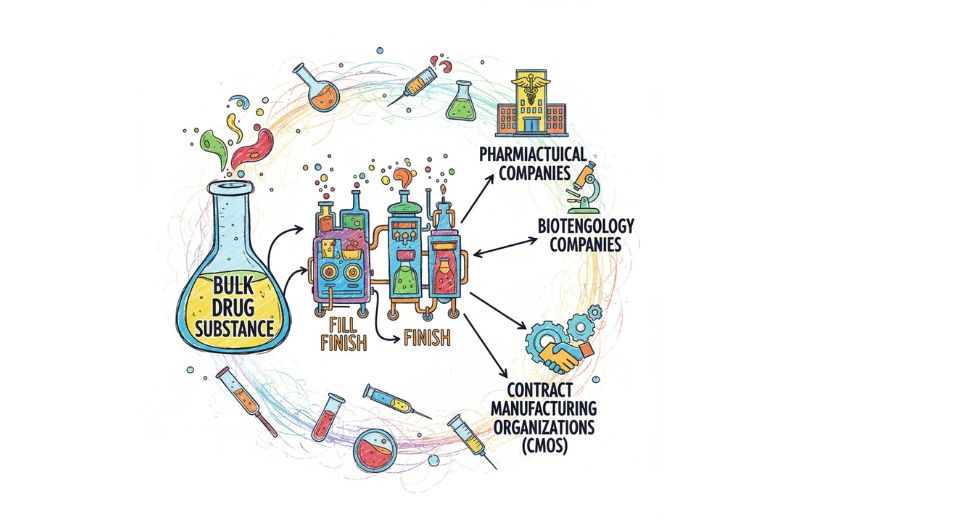
Every time a vaccine, biologic therapy, or injectable medication comes to the clinic or pharmacy, it has already gone through the last and most important stage of filling, sealing, and packaging. Metastat Insight presents the global fill finish manufacturing market, which draws attention to this phase that is very important. The medicines are turning to be more targeted, more complex and more sterile, thus the need for high-precision fill-finish production is coming into sharper focus than ever before.
Grasping the Market Backdrop
The pharmaceutical and biotech companies are under increasing pressure to deliver the therapies to the patients quicker and cheaper. The challenges of the drug complexity, making medicine for each patient, keeping sterility at the highest standards, and the instabilities in the global supply chain are just a few of the problems. The fill-finish market, in that difficult situation, plays an enabling role: it transforms the active pharmaceutical ingredients into final containers (vials, cartridges, prefilled syringes) in very controlled conditions, ready for shipping and use. By either outsourcing or upgrading their capabilities, companies are able to directly address the issues of time-to-market, scale-up bottlenecks, and facility modernization.
What the production step involves and the value it brings
The fill-finish manufacturing market at its very essence consists of the operations related to sterile filling (aseptic or closed?vial systems), sealing, inspection, packaging and labeling of injectable drug products. It is the first step in the production process where the drug gets pavilioned and reaches the market in the right way. Among the main pros are the assurance of sterility, the deterrence of contamination, and the possibility of small-scale production or flexible runs which is very important for personalized or niche therapies. Additionally, the use of automation and robotics is a major factor in enhancing throughput and consistency. For instance, as there is an increase in the development of biologics, biosimilars and cell-therapy bags, the fill-finish phase has to undergo a change in order to cater to the new container types, lower volumes, complexities and the bespoke workflows that are at work. The value derives from the transformation of a drug candidate into a properly packaged product that is reliable and meets regulatory and quality requirements while also being fast-tracked for commercialization.
How the landscape has evolved
Fill-finish operations were in the past mainly carried out in-house by large pharmaceutical manufacturers or basic contract manufacturers. In the last ten years, outsourcing of fill-finish services, modular cleanrooms, and automated fill lines, which are the main products of the innovation strata, have become quite common. Some of the most prominent steps include the global vaccination production industry that was able to respond rapidly during global health crises, thus unfolding the constraints of outdated fill-finish capacity and consequently, the advantages of modern lines and new container formats. The emergence of the biologic and gene therapy markets has imposed tougher standards for the fill-finish capabilities and thus, innovations like robotic handling, single-use systems, integrated inspection, and real-time monitoring have all come up as a result. These advancements have further secured the position of the fill-finish manufacturing market as a vital component of the drug supply chain.
Where adoption is strongest and emerging geographies
The current major markets of the global fill-finish manufacturing market are North America and Europe. The regulatory frameworks in those regions, coupled with the development of advanced biotech ecosystems and capital-intensive manufacturing, have led to the dominance of the global fill-finish market. For instance, the majority of the contract manufacturing organizations (CMOs) which provide fill-finish services are situated in the United States and Western Europe. On the contrary, the Asia Pacific region, which includes countries like India, China, and South Korea, is emerging as a strong player in the market, driven by the factors of increasing domestic pharmaceutical production, cost advantages, and growing export-oriented manufacturing. In Latin America and some parts of the Middle East, the emergence of the global fill-finish capacity trend is being viewed as a sign of potential. In this way, the global fill-finish manufacturing market is characterized by both strongholds and fast-moving new geographies.
Obstacles and fresh opportunities
There are still multiple challenges confronted by the industry. The high capital costs, the need for sterility and strict compliance with regulations, the validation of the container-closure systems, the lack of skilled workforce, and the disruptions in the supply chain (e.g., of critical consumables) are all barriers. On the other hand, there are numerous opportunities such as advanced fill-finish lines for cell and gene therapies, automation, and digitalization, proximity manufacturing (near patients or regional hubs), flexible small-batch formats, and synergies with adjacent segments like biologics drug-substance manufacturing or cold-chain packaging. In other words, although complexity and cost present challenges, innovation and market expansion are creating new avenues.
Why the moment is now
The global medical landscape is shifting therapies are more complex, regulatory demands higher, and supply-chain resilience more important than ever. The global fill finish manufacturing market presented by Metastat Insight arrives at a time when pharma and biotech must combine speed, quality and flexibility. By enabling safe and reliable final dosing formats for the next generation of treatments, this market holds a central place in shaping healthcare’s future.
Drop us an email at:
Call us on:
+1 214 613 5758
+91 73850 57479