
Nov 12, 2025
The pressure for faster, more confident medical decisions has increased the interest in advanced diagnostic methods. Metastat Insight global chemiluminescence immunoassay market is a good representation of this trend, showing that healthcare professionals and lab personnel are continually looking for fast and clear solutions during the evaluation of diseases. Each specimen holds priceless biological secrets, but the traditional methods of testing usually give delayed answers. Chemiluminescence immunoassay offers a revolutionary approach, where even the smallest amounts of biological markers are rendered visible by the controlled generation of light. The awareness of disease progression, therapeutic response, and preventive screening now relies heavily on reliable detection at the early stages. Modern healthcare practices demand precision that is delivered in an efficient manner. This market signifies a step towards laboratory efficiency, innovation in the evaluation of immune responses, and an overall increase in clinical confidence.
Market Context
The healthcare sector is facing a major diagnostic challenge due to the ever-increasing number of tests performed, the emergence of new pathogens, and the need for rapid result delivery. The delay in reporting from traditional methods can disrupt the treatment decision-making process. The complexity of the workflow and the increase in the number of tests in clinical laboratories are driving the adoption of technology that allows for automation and at the same time reduces the manual steps involved. Chemiluminescence immunoassay instruments are capable of supporting the processing of high volumes of specimens, thus allowing laboratories to cater to the requests for testing without compromising on the quality of the analysis. The increasing use of specialized biomarker tests during regular health checkups is now making it possible for the deployment of such platforms on a larger scale.
How the Technology Works / Why the Process Delivers Value
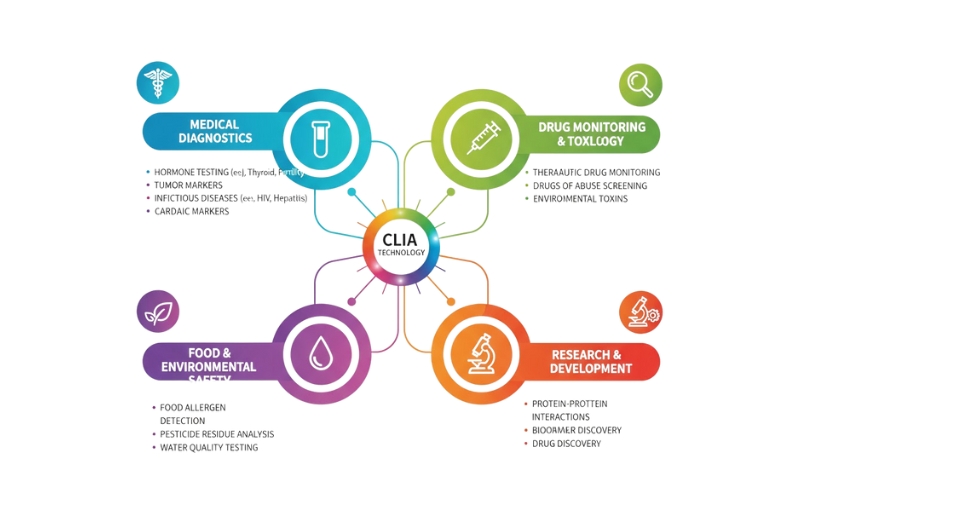
The chemiluminescence immunoassay technology being mentioned here is based on the principle of light emission due to a chemical reaction to reveal the presence of the target molecules. Enzyme-labeled antibodies are the molecules that are playing this interaction between the biological analytes; the light generated as a product of this activation reaction is what is being measured. The amount of light emitted indicates the amount of the target molecule present, allowing for detection of very low levels. The best analytical performance has made it possible to test for hormones, heart attack indicators, infections and many more. The combination of careful calibration and system automation minimizes human error, while the list of available tests keeps growing.
The laboratory heads are often pointing out the benefits of increased automation and less maintenance. The Immunoassay machines will not only perform the analysis but also prepare it for the next round of samples and consumables all day long, thus securing the lines of work that are uninterrupted. The same technology can easily be connected to the laboratory information systems, thus enhancing the digital traceability and audit readiness. The faster performance leads to better patient management and shorter hospital stays, which is a positive impact on the healthcare system's efficiency overall.
Growth Story / Technological Evolution
The chemiluminescence immunoassay technology has changed from being used in specialized research places to being used in clinical routine widely. The systems of the early generation mainly highlighted their sensitivity advantages. After that, the hairstyling increased automation, consumable stability, assay speed, and user interface quality. The instrument configurations have a wide range now, from compact benchtop units for urgent care settings to fully automated suites for large centralized laboratories.
The latest development includes not only better reagent consistency but also better signal detection, and that comes hand in hand with precision in low-abundance analyte identification. Continuous improvements within assay design expand testing menus, enabling healthcare facilities to perform multiple types of analysis on the same platform. The chemiluminescence immunoassay technology is already incorporated into the diagnostic categories of new disease biomarkers that have gained global awareness. This ongoing transformation of chemiluminescence immunoassay technology strengthens its adoption in hospitals, independent reference laboratories, and community clinics.
Regional and Global Adoption Patterns
Regulatory frameworks, laboratory infrastructure investment, and clinical awareness are the main reasons for the difference in adoption rates globally. Traditional health care systems prefer high-volume testing and automation, thus facilitating the quick installation of chemiluminescence immunoassay systems. Healthcare modernization regions are adopting this technology because it is capable of transferring advanced diagnostics to mid-sized and regional hospitals.
In the case of a newly developing market, a larger investment in public health screening programs leads to an interest in scalable laboratory solutions. Hospital networks are looking for a system that will ease their operational burden and at the same time allow them to conduct large population screening programs. The continuous improvement of healthcare reimbursement policies and the expansion of private diagnostic chains are some of the factors that contribute to the strong adoption momentum in the market. The market players' response is to offer localized service networks and education programs for lab personnel support.
Challenges and Opportunities
Despite widespread progress, adoption barriers remain. Acquisition costs, integration planning, and the need for skilled laboratory staff can influence purchasing timelines. Regulatory requirements may extend evaluation cycles before approval and installation. Smaller laboratories may question return on investment if daily volumes fluctuate.
Opportunities arise from ongoing innovation in reagent design, software enhancements, and expanded biomarker panels. Integration with digital diagnostic ecosystems positions chemiluminescence immunoassay technology for collaboration with telehealth and remote monitoring programs. Machine learning and data analytics support predictive maintenance, reducing downtime and improving resource utilization. Broader disease awareness campaigns create demand for additional tests, encouraging manufacturers to explore new clinical indications.
Why This Market Matters Now
Healthcare systems worldwide aim to shift from reactive treatment toward proactive prevention. Chemiluminescence immunoassay technology directly supports this transition by revealing disease indicators earlier, enabling healthcare professionals to respond at the right moment. Increased focus on personalized treatment strategies depends on reliable biomarker data produced without unnecessary delays. Sustainability initiatives in laboratories also benefit from reduced reagent waste, efficient energy use, and minimized repeat testing. Digital transformation across healthcare complements automated diagnostic systems, reinforcing interoperability and continuous data documentation.
The global chemiluminescence immunoassay market presented by Metastat Insight symbolizes a shift toward confident decision-making and rapid access to actionable clinical information. Continuous innovation, broader accessibility, and strong integration with future healthcare models ensure ongoing relevance as diagnostics shape outcomes across global populations.
Drop us an email at:
Call us on:
+1 214 613 5758
+91 73850 57479