MARKET OVERVIEW
In an era where exactitude and early detection reign supreme, Global Herpes Simplex Virus (HSV) Testing Kit market has ascended to prominence, addressing the critical imperative of identifying and managing the herpes simplex virus, a ubiquitous and enduring health quandary.
Herpes Simplex Virus, frequently abbreviated as HSV, stands as a viral affliction that manifests in multifarious guises, primarily as HSV-1 (oral herpes) and HSV-2 (genital herpes). Both iterations of this viral entity can induce agonizing and recurrent lesions, and the highly contagious nature of the virus amplifies its significance as a paramount public health conundrum. The efficacious management and punctual diagnosis of HSV infections emerge as indispensable prerequisites for mitigating suffering and forestalling further transmission.
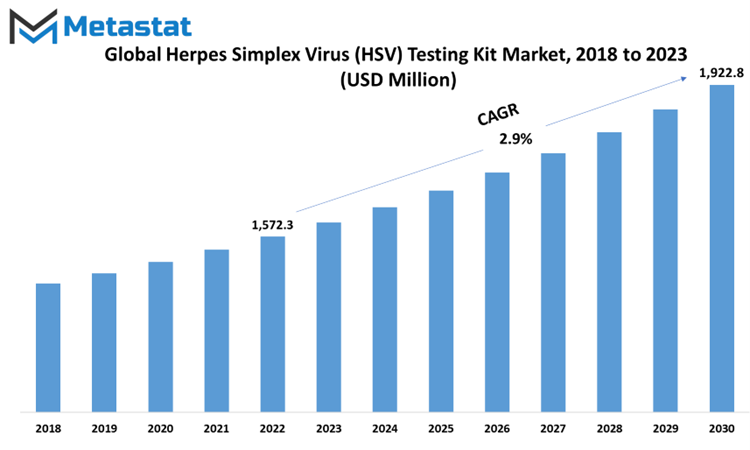
Within this context, the pivotal function of HSV testing kits becomes palpable. These kits are meticulously crafted to discern the presence of HSV within patient specimens, endowing healthcare practitioners with a dependable modality for ascertaining the infection. Such examinations not only prove instrumental in corroborating the diagnosis but also serve as a means of distinguishing between the two HSV variants, an imperative differentiation with profound implications for individualized therapeutic interventions. Global Herpes Simplex Virus (HSV) Testing Kit market is estimated to reach $1,922.8 Million by 2030; growing at a CAGR of 2.9% from 2023 to 2030.
MARKET DYNAMICS
Increasing prevalence of HSV infections worldwide and Growing demand for rapid and accurate diagnostic tests for HSV are key driving factors of the market. In recent epochs, the global landscape has borne witness to a disconcerting surge in the prevalence of Herpes Simplex Virus (HSV) infections, an infamous viral affliction known for its recurrent and occasionally incapacitating manifestations. This worldwide health quandary has ignited an urgent imperative for expeditious and pinpoint diagnostic methodologies to arrest transmission and alleviate the repercussions of HSV. Consequently, the HSV testing kit market has borne witness to a substantial resurgence, driven by sundry pivotal factors.
A paramount impetus behind the burgeoning HSV testing kit market is the escalating ubiquity of HSV infections on a global scale. HSV infections, stemming from herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2), possess an exceptional capacity for contagion and can precipitate a spectrum of clinical manifestations, encompassing agonizing oral and genital ulcers. The facile transmission dynamics and the dearth of a definitive cure accentuate the imperativeness of precise and timely diagnosis. The surging tally of documented cases has necessitated a robust and streamlined diagnostic infrastructure, underpinning the sustained clamor for testing kits.
Moreover, the burgeoning demand for expeditious and precise diagnostic modalities for HSV has cast a spotlight upon this niche. In a realm where judicious and timely diagnosis can engender a profound impact on patient outcomes, healthcare professionals are progressively leaning on diagnostic instruments that yield swift and precise results. HSV testing kits, endowed with the capability to furnish outcomes within moments, have emerged as indispensable instruments in the arsenal of medical practitioners. This escalating demand has not only catalyzed fiscal influx but has also ushered in an era of innovation in diagnostic technology.
Nonetheless, amid the optimism encircling the HSV testing kit domain, certain formidable impediments loom large, casting shadows on its growth trajectory. One hurdle is the in regard to the awareness and underdiagnosis concerning HSV infections. Despite the virus's wide-reaching prevalence, a substantial cohort of the afflicted populace remains undiagnosed. This underdiagnosis is frequently ascribed to the asymptomatic presentation of HSV infections in some individuals and a general paucity of cognizance pertaining to the virus. Bridging this awareness chasm and elevating diagnostic rates presents an imposing challenge.
Furthermore, the costs entwined with HSV testing kits and the constrictive reimbursement policies operative in myriad healthcare systems have erected fiscal barriers for patients in pursuit of testing and therapeutic interventions. The fiscal dimension, concomitant with the lack of comprehensive reimbursement protocols, has spawned a milieu wherein access to diagnostic resources may be curtailed for discrete demographic segments. This incongruity in access to diagnostic assets is an issue that mandates discerning scrutiny.
Nonetheless, among these challenges, the HSV testing kit market is opportunities. The development of point-of-care HSV testing kits, to be user-friendly and deployable in resource-constrained environs, stands as a beacon of optimism. Such innovations harbor the potential to transfigure the sphere of HSV diagnostics by extending the purview of testing to marginalized regions and demographic strata. Through the democratization of accurate HSV diagnosis, these strides have the capacity to not only propel market expansion but also play a pivotal role in the global crusade against this viral scourge.
MARKET SEGMENTATION
By Type
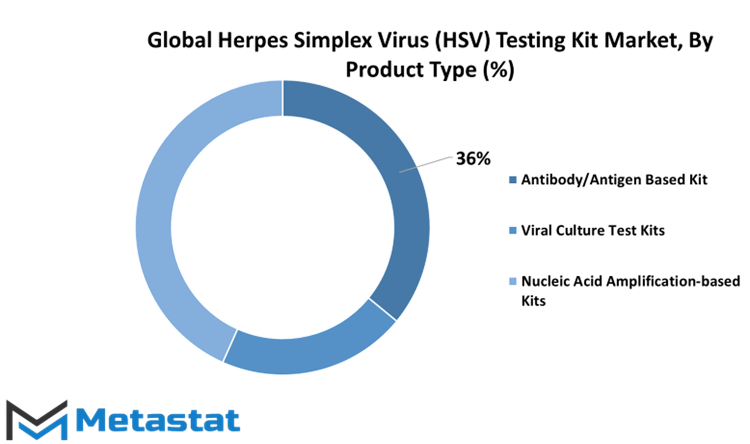
Within the diagnostic testing, the categorization of test kits is crucial for precision and efficacy. In this vein, the segmentation of diagnostic test kits encompasses three distinct and highly specialized types, each designed with a specific purpose and mechanism in mind. These segments are Antibody/Antigen Based Kits, Viral Culture Test Kits, and Nucleic Acid Amplification-based Kits.
Antibody/Antigen Based Kits, constitutes a pivotal arm of diagnostic testing. These kits are predicated on the detection of antibodies or antigens within the patient's biological sample. Antibodies are the body's natural defense mechanism against foreign invaders such as viruses, and their presence can indicate a prior infection or an ongoing immune response. Conversely, antigens are molecules found on the surface of pathogens, acting as identifiers for the immune system to recognize and combat them. These kits are particularly instrumental in the diagnosis of infectious diseases where the body mounts an immune response. They offer rapid results and are frequently employed in situations requiring quick detection, such as in the case of a contagious disease outbreak and it will be 691.2 USD Million in 2030.
On the other hand, Viral Culture Test Kits occupy a distinct niche within the diagnostic toolkit. These kits facilitate the isolation and subsequent growth of viruses in a controlled laboratory environment. By nurturing the proliferation of viruses, scientists and healthcare professionals gain invaluable insights into the nature of the pathogen, its virulence, and its susceptibility to antiviral treatments. This segment is pivotal in research settings, epidemiological studies, and the development of novel therapies. It allows for the comprehensive analysis of the virus's behavior and characteristics, serving as a cornerstone for in-depth investigations and it accounted for 20.8% of Herpes Simplex Virus (HSV) Testing Kit Market in 2022
The Nucleic Acid Amplification-based Kits constitute a sophisticated and highly precise category of diagnostic tools. The Nucleic Acid Amplification-based Kits segment was valued at 662.3 USD Million in 2022. These kits rely on the amplification of the virus's genetic material, typically its RNA or DNA, to detect its presence with unparalleled sensitivity. Polymerase Chain Reaction (PCR) is a prime example of such amplification techniques. These kits are renowned for their accuracy and are often considered the gold standard in diagnostic testing. They excel in identifying viruses even in the early stages of infection when viral loads are low. Nucleic Acid Amplification-based Kits are indispensable in confirming diagnoses, monitoring disease progression, and ensuring the efficacy of antiviral treatments.
By End Use/Application
The global Herpes Simplex Virus (HSV) Testing Kit market, a fact that categorically influences its dynamics is the segmentation based on end use/application. This segmentation strategy effectively delineates the diverse arenas where these testing kits find relevance and utility. In this essay, we delve into a comprehensive exploration of each segment type, elucidating the distinctive characteristics and implications associated with Diagnostic Centers, Hospitals, Home Use, and the broader category of Others.
Diagnostic centers stand as veritable pillars of healthcare, serving as crucibles for the accurate identification and diagnosis of various medical conditions, including herpes simplex virus infections. These specialized facilities are renowned for their cutting-edge diagnostic equipment and highly trained personnel, ensuring precision in testing and diagnosis. In the context of HSV testing kits, diagnostic centers play a pivotal role in facilitating prompt and accurate detection. Their professional expertise and state-of-the-art infrastructure make them particularly adept at conducting comprehensive tests, often with a rapid turnaround time. Moreover, diagnostic centers cater not only to individuals seeking clarity on their health status but also offer indispensable support to healthcare practitioners in corroborating clinical assessments. This segment embodies the essence of precision and reliability, setting a benchmark for HSV testing kit applications.
Hospitals, the bastions of medical care, encompass a vast spectrum of healthcare services, including the diagnosis and management of infectious diseases like herpes simplex virus infections. Within the precincts of hospitals, HSV testing kits assume a pivotal role in patient care. Hospitals typically employ these kits as part of their diagnostic arsenal, aiding in the swift identification of herpes infections, thereby guiding treatment decisions. Hospitals, with their multidisciplinary teams of healthcare professionals, rely on the timely and accurate results provided by these kits to formulate treatment regimens tailored to individual patients. The hospital segment signifies the integration of HSV testing kits into the broader landscape of healthcare, where their contributions are instrumental in ensuring patient well-being.
The advent of technology and advancements in the field of diagnostics have democratized healthcare to a significant extent. The segment of home use for HSV testing kits epitomizes this democratization. These kits empower individuals to take charge of their health by offering the convenience of testing from the comfort of their homes. While ensuring privacy and convenience, home-use HSV testing kits are designed for user-friendliness, often accompanied by clear instructions. Users can collect samples and obtain results without the need for specialized medical training or assistance. This segment underscores the empowerment of individuals in managing their health proactively and discreetly, marking a paradigm shift in healthcare accessibility.
The category of others within the realm of HSV testing kit applications is a dynamic and diverse classification, encompassing a range of settings and contexts that do not fit neatly into the predefined categories of diagnostic centers, hospitals, or home use. This catch-all segment highlights the adaptability and versatility of HSV testing kits. It includes settings such as clinics, community health centers, and specialized healthcare facilities that may not conform to the traditional classifications but nevertheless rely on these kits for their diagnostic needs. This segment is a testament to the wide-reaching impact of HSV testing kits across various healthcare contexts, transcending conventional boundaries.
REGIONAL ANALYSIS
The geographical segmentation, the global market for Herpes Simplex Virus (HSV) Testing Kits undergoes a partition into several key regions, each wielding its own distinctive share of this critical market landscape. As of the year 2022, North America assumes a commanding position, staking claim to a substantial 35.9% of the overall Herpes Simplex Virus (HSV) Testing Kit market.
Further, scrutinizing North America's dominion, it is dissected into three discernible constituents: the United States, Canada, and Mexico. Meanwhile, on the European front, the outlook is no less noteworthy. Europe, as a collective entity, charts a course toward attaining a market valuation of 618.3 USD Million by the year 2030, reflecting a forecasted trajectory of significant growth. The European terrain, in turn, encompasses the United Kingdom, Germany, France, Italy, and the Remaining Expanse of Europe.
Venturing forth to the Asia-Pacific region, we encounter a mosaic of vibrant economies. This region's segmentation entails the distinct identities of India, China, Japan, South Korea, and the Remaining Territories within Asia-Pacific. South America, another integral piece of this global puzzle, consists of the vibrant nations of Brazil and Argentina, along with the Remaining Uncharted Territories within South America.
This geographical segmentation includes the dynamic tapestry of the global Herpes Simplex Virus (HSV) Testing Kit market, delineating its prevalence and potential across North America, Europe, Asia-Pacific, South America, and the Middle East & Africa.
COMPETITIVE PLAYERS
Prominent players within the Herpes Simplex Virus (HSV) Testing Kit sector encompass Abcam plc, known for its collaboration with esteemed academic institutions, research entities, and industry partners, which positions it as a frontrunner in pioneering scientific advancements and contributing to groundbreaking discoveries across diverse domains. Additionally, Abcam exhibits a steadfast commitment to corporate social responsibility and sustainability. bioMérieux SA, Thermo Fisher Scientific Inc., and F. Hoffmann-La Roche Ltd. are other influential entities in this domain. Roche, through its Pharmaceuticals and Diagnostics divisions, occupies the vanguard of medical progress, offering innovative therapies, precision diagnostics, and tailored healthcare solutions. Roche's dedication to research and development, customer support, and corporate social responsibility underscores its ongoing contributions to advancing medicine and enhancing global health. A diverse array of companies, including DiaSorin S.p.A., Quidel Corporation, Luminex Corporation, QIAGEN, Norgen Biotek Corp., Hologic, Inc., Bio-Rad Laboratories, Inc., Meridian Bioscience Inc., Teco Diagnostics, Maruho Co., Ltd., Virusys Corporation, GeneProof, AdvaCare Pharma, Genome Diagnostics Pvt. Ltd., InTec PRODUCTS, INC., and Creative Diagnostics, further enrich the tapestry of the HSV Testing Kit industry with their distinctive expertise and contributions.
Herpes Simplex Virus (HSV) Testing Kit Market Key Segments:
By Type
- Antibody/Antigen Based Kit
- Viral Culture Test Kits
- Nucleic Acid Amplification-based Kits
By End Use/Application
- Diagnostic Centers
- Hospitals
- Home Use
- Others
Key Global Herpes Simplex Virus (HSV) Testing Kit Industry Players
- Abcam plc
- bioMérieux SA
- Thermo Fisher Scientific Inc.
- Hoffmann-La Roche Ltd.
- DiaSorin S.p.A.
- Quidel Corporation
- Luminex Corporation
- QIAGEN
- Norgen Biotek Corp.
- Hologic, Inc.
- Bio-Rad Laboratories, Inc.
- Meridian Bioscience Inc.
- Teco Diagnostics
- Maruho Co., Ltd.
- Virusys Corporation
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential






 US: +1-(714)-364-8383
US: +1-(714)-364-8383






