MARKET OVERVIEW
The global decentralized clinical trials (DCT) market is leading the way for a new trend in the pharmaceutical and life sciences sector, providing a different framework for how clinical studies will be undertaken across geographies. The market will evolve around solutions that enable sponsors, investigators, and participants to engage with trial protocols using digital technologies, remote monitoring software, and home-based processes. Instead of depending on centralized research sites, the model will spread data collection, patient recruitment, and clinical evaluations to wider, frequently more convenient settings. What characterizes the global decentralized clinical trials (DCT) market is its focus on convenience, technological capabilities, and flexibility of research models to actual participants' environments.
Rather than needing to make multiple visits to particular trial sites, participants will be enrolled, monitored, and followed up on by using secure virtual channels, mobile health technology, and local healthcare providers. This change will not only impact how trials are conducted, but also who sponsors and contract research organizations (CROs) choose and oversee sites, recruit participants, and assess results. The market will thereby redefine interactions among stakeholders and place logistics, operation, and regulatory innovations at the center. Major players in the pharmaceutical and biotech industries will pursue this strategy to minimize reliance on geographic location and enhance diversity in patient populations. The global decentralized clinical trials (DCT) market will make it possible for patient convenience and protocol compliance to exist simultaneously.
Through digital health records, electronic patient-reported outcomes, and wearable devices, trial data will be collected in near real-time to facilitate quicker decision-making and adaptive trial designs. The technologies facilitating this change will include video consultations, cloud-based data repositories, mobile diagnostics, and AI-powered platforms. As regulators, sponsors, and the market grow, they will be required to walk the fine line between compliance and innovation. The global decentralized clinical trials (DCT) market will be confronted by changing needs related to data integrity, patient confidentiality, and technological validation. They will impact digital platforms' certification, how remote assessments are validated by investigators, and how remotely informed consent is provided by patients.
Data safety and interoperability will also emerge as key aspects since global trials demand smooth consolidation of digital inputs from various geographies. Pharmaceutical companies and research centers will also realign their alliances to enable decentralized models. Suppliers of remote technology, logistics assistance, and home-health services will be key to providing trial results that are acceptable from a clinical endpoints perspective. The global decentralized clinical trials (DCT) market will create new opportunities for local clinics and healthcare workers to play bigger roles in trials that were previously reserved for high-investment research sites. This redistribution of clinical practice will also add to a broader geographic reach of research, particularly in underprivileged or rural regions.
In the future, the global decentralized clinical trials (DCT) market will serve as a basis for an increasingly integrated, technology-enabled research infrastructure. It will shape the way clinical evidence is gathered and disseminated, how operational planning is done, and how patients engage in research. As digital maturity evolves within healthcare systems, this model will continue to shape clinical research in ways that are quantifiable and transformative globally.
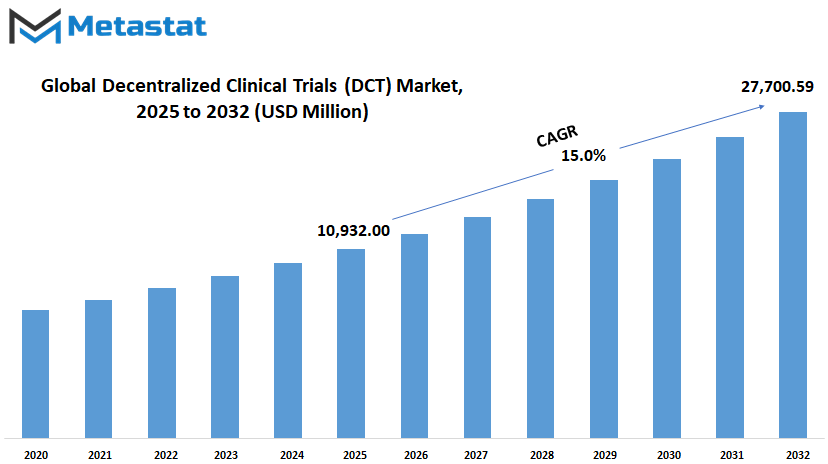
Global decentralized clinical trials (DCT) market is estimated to reach $27,700.59 Million by 2032; growing at a CAGR of 15.0% from 2025 to 2032.
GROWTH FACTORS
The global decentralized clinical trials (DCT) market is slowly redefining the way clinical research is conducted. In contrast to traditional studies dependent on central locations and inflexible processes, this trend focuses more on accessibility and flexibility. Perhaps one of the primary reasons the model is taking off is due to the growing demand for patient-centered approaches. Individuals these days want to join studies without needing to go too far or quit their jobs. The change has benefited both participant recruitment and retention. By simplifying how individuals join and stay in studies, researchers are now able to obtain better data in the long term. Moreover, advances in digital health technologies enabled remote tracking of health information.
Wearable technology, mobile applications, and telemedicine platforms enable researchers to gather data in real time, without the need to visit medical facilities repeatedly. Not only does this ease the participants' burden, but it also provides a more accurate picture of their health for longer durations. With better and timelier data, findings quality increases, and decisions are quicker. All these technological advancements enable the global decentralized clinical trials (DCT) market to expand further by rendering studies more efficient and inclusive. But there are issues that still require concern. The adoption of remote tools raises significant questions regarding data safety.
As sensitive health information is shared digitally, the potential for breaches increases. Ensuring personal details are safeguarded is critical to ensuring trust between participants and researchers. Any shortfall in cybersecurity would hinder the adoption of this model. In addition to this, regulatory and logistical hindrances remain in the way. Varying countries have different regulations regarding how trials ought to be run, and coordination across multiple regions can become problematic. This places layers of complexity that must have close planning and regular updating to stay compliant.
In the future, the global decentralized clinical trials (DCT) market is promising, particularly when one considers underserved regions. Remote engagement provides opportunities for individuals in emerging economies to participate in trials to which they had no prior access. This greater reach can be used to provide research that covers diverse populations, which is necessary for treatments to work across diverse groups. These advancements, implemented with the correct strategies, have the potential to advance the market, providing more favorable results for participants and researchers alike.
MARKET SEGMENTATION
By Study Type
The global decentralized clinical trials (DCT) market is gradually transforming the manner in which medical research is carried out. Traditional clinical trials have always been geographically based, with patients needing to travel to a specific center to take part. This process, although useful in its day, is not without its disadvantages. Distance, fare, and busy schedules deter many willing volunteers. Decentralized trials dismantle such barriers by enabling participants to participate in research from home using digital channels. The shift comes with the benefit of ease and opens up participation to a wider number of people, improving the quality and validity of results. The future for the market seems to increasingly depend on remote methods.
With the growth of digital health technologies, such as mobile applications and wearable devices, data collection in real-time becomes a possibility. This means researchers will be getting updated data continuously and won't need to wait for site visits. These technologies also help with keeping patients engaged throughout the study, leading to better results and fewer dropouts. With growing faith in internet-based platforms, more and more healthcare professionals and sponsors will choose decentralized methods over traditional ones. The market can be viewed by various types of studies. Interventional studies, employed primarily to contrast new treatments, can benefit significantly from this model.
By the direct method of accessing patients at home, researchers can monitor the performance of treatments in real-life settings. This may help give a clearer image of what a drug or treatment does outside of clinical environments. Observational research, in which patients are observed without changing their usual care, also suit the decentralized model. They rely strongly on accurate data collection, and computing units help and make it more reliable. Expanded Access, a trial in which patients are allowed to receive an as-yet-unwisely-disseminated treatment, will also be easier to implement remotely. It means faster help for patients in urgent need. The global decentralized clinical trials (DCT) market will continue to grow in the years ahead as both technology and public acceptance of digital health climb. What used to take months to plan and strictly follow detailed schedules can now be achieved with a click of the mouse and watching from afar. This will bring about faster and more varied research and unveil new paradigms for understanding health among different communities.
By Component
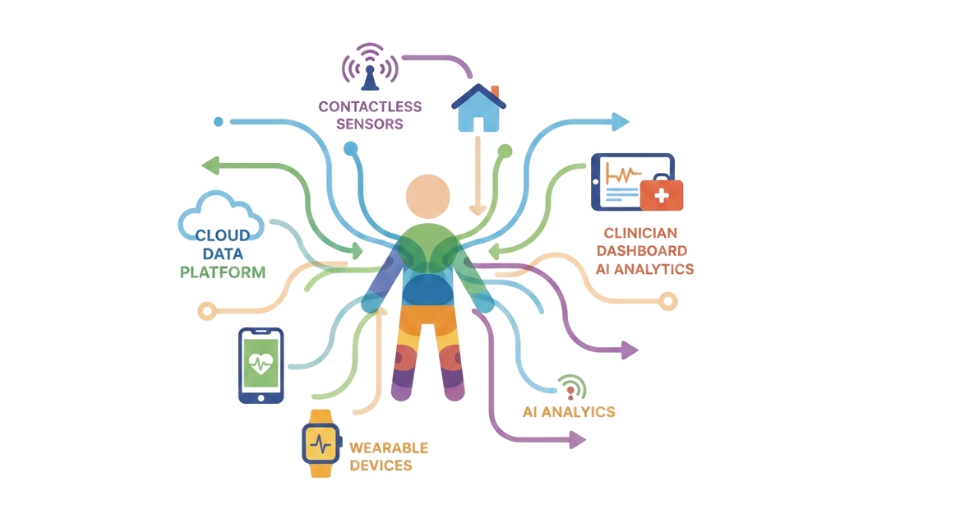
The global decentralized clinical trials (DCT) markets are gradually shifting the way medicine is researched. With technology on the rise for many sectors, the medical world is also evolving to provide easier and more effective clinical trials. Rather than being asked to sit through lengthy transportation or show up for in-office appointments, decentralized trials enable the majority of procedures to take place remotely. This change is opening up more prospects for individuals to get involved in trials as well as allowing researchers to gather better and more precise data. In the future, the services and devices that enable decentralized trials will only become more significant.
Wearable devices, for example, are already assisting in gathering heart rate, activity level, and sleep patterns data in real-time. These products facilitate easier monitoring of participants without interfering with their daily lives. As they continue to develop, the data they yield will become more valuable for researchers seeking accurate data without having to exclusively depend on planned visits or self-reported data. The other key segment in this market is eConsent solutions. These enable participants to provide consent to participate in a study via electronic means instead of via paper forms.
This not only saves time but also simplifies the presentation of the finer points of a study via videos or interactive mediums. In the future, developments in these tools will most likely result in better comprehension among participants, which assists in building trust and enhancing the quality of studies as a whole. Telemedicine platforms also have a major role to play in facilitating decentralized trials. They enable participants to communicate with physicians or researchers using video calls. This allows individuals from various areas to participate in trials that previously they might not have been able to access. With the continuous improvement of internet access and devices becoming cheaper, telemedicine will bridge the gap between researchers and potential participants. Data management software holds it all together. Data collected from devices, virtual consultations, and consent forms must be stored and organized properly.
This software facilitates improved tracking, enhanced protection of privacy, and faster analysis of data. When more tools get linked, software systems will have an increasingly larger role in keeping things running efficiently. With these expanding elements, the global decentralized clinical trials (DCT) market will probably experience steady growth. It will continue to assist in reshaping clinical research in more inclusive, efficient, and flexible ways, hinting at a future where innovation goes hand in hand with care.
By Application
The global decentralized clinical trials (DCT) market is headed towards a future where patient convenience and technology become the key. Rather than requesting that individuals go to research sites repeatedly, additional studies will be created to enable participation from the comfort of their homes. It's not simply about convenience; it's also about engaging individuals who otherwise might be excluded due to distance, physical disabilities, or hectic lives. With the removal of the transportation requirement, more people can be included in studies by researchers, increasing the quality and applicability of the data they gather. As the healthcare sector continues to open up to digitalization, decentralized trials will be the go-to method for most studies.
Equipment that will allow health to be monitored remotely, software programs that gather patient input in real time, and secure systems that process data without requiring face-to-face contact will all be included in this transformation. These technologies won't eliminate human contact completely, but they will decrease the frequency with which patients and physicians must see each other. Eventually, this might accelerate how fast new treatments are being tested and approved. Various aspects of the medical field will be touched by this expansion individually. In the field of oncology, in which patients frequently endure long and arduous treatment processes, the ability to remain at home more frequently can act as a stress reducer and comfort enhancer.
In cardiology, with the importance of constant monitoring, wearable technology will provide physicians with an uninterrupted flow of meaningful data. Neurology research, which tends to involve extended periods of observation, will benefit from digital technologies that monitor behavior and reactions over time. For respiratory diseases, particularly those that can flare up unpredictably, real-time monitoring can enable researchers to respond more quickly and precisely. The success of the global decentralized clinical trials (DCT) market will also hinge on how effectively companies manage data safety and patient privacy. As more personal data is exchanged using digital means, security will be an ongoing concern. Nevertheless, as technology continues to evolve, patients will increasingly trust these platforms, and it will become simpler for them to agree to participate in a trial.
In the future, the integration of human care and digital care will be the new normal. This will not only enhance the way clinical trials are conducted but also the way care is delivered in general, with potential for quicker discoveries and improved access to treatment around the globe.
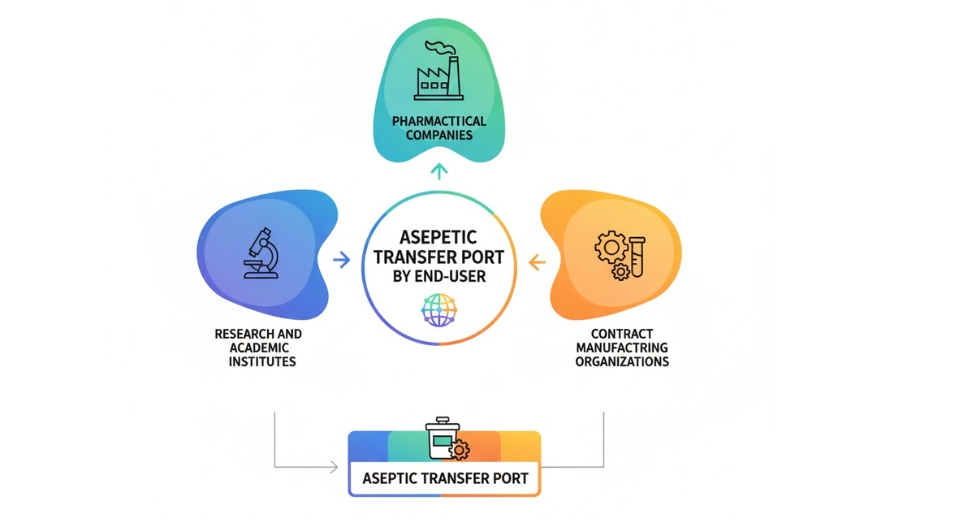
By End-User
The global decentralized clinical trials (DCT) market gradually becomes an indispensable component of how the future is going to resemble in research and drug development. With conventional clinical trials having access limitations by geographical location, pricing, and by access to population, this fresh approach presents an open and inclusive means to achieve data collection as well as assessing new treatments. Growth of this model indicates a bold shift towards enriching the participants' experience and ensuring research is still valid. In this design, rather than bringing participants into one location, the research is conducted using electronic tools, at-home visits, and neighborhood healthcare providers. Not only does it enable more access, but smoother data collection can also be made possible. In the future, participants in this market such as pharma companies, biotechnology companies, contract research organizations, and academic and research institutions will probably have an even bigger role to play.
Pharma companies will be able to speed up testing, reduce costs, and reach more diverse populations using these trials. By becoming more patient-focused in trials, these companies might eliminate dropout rates and achieve improved outcomes. Biotechnology companies will be the same. Because some of them are niche therapies and new therapies, they can especially benefit from this model in being able to access people who require highly specialized care or are located in distant regions. Contract research organizations will be a significant component of this framework. These organizations usually coordinate trials for other firms, and their flexibility will be challenged.
To stay competitive, they will likely invest more in digital platforms, remote monitoring tools, and secure data handling. Their success may depend on how well they can manage these trials without needing to gather everyone in one place. Academic and research institutions also have a part to play. These organizations can help refine this method by studying its effects, offering insights, and improving how trials are done. In the future, we may witness greater cooperation among these various end-users. As technology and tools continue to evolve, each organization will likely adapt the way it operates to keep pace. There will be new jobs, additional sharing of information, and better methods for managing patient data.
This transformation may not only change the way trials are conducted but also how treatments find their way to the public. The global decentralized clinical trials (DCT) industry will probably continue to increase as these trends gain traction.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$10,932.00 million |
|
Market Size by 2032 |
$27,700.59 Million |
|
Growth Rate from 2025 to 2032 |
15.0% |
|
Base Year |
2024 |
|
Regions Covered |
North America, Europe, Asia-Pacific, South America, Middle East & Africa |
REGIONAL ANALYSIS
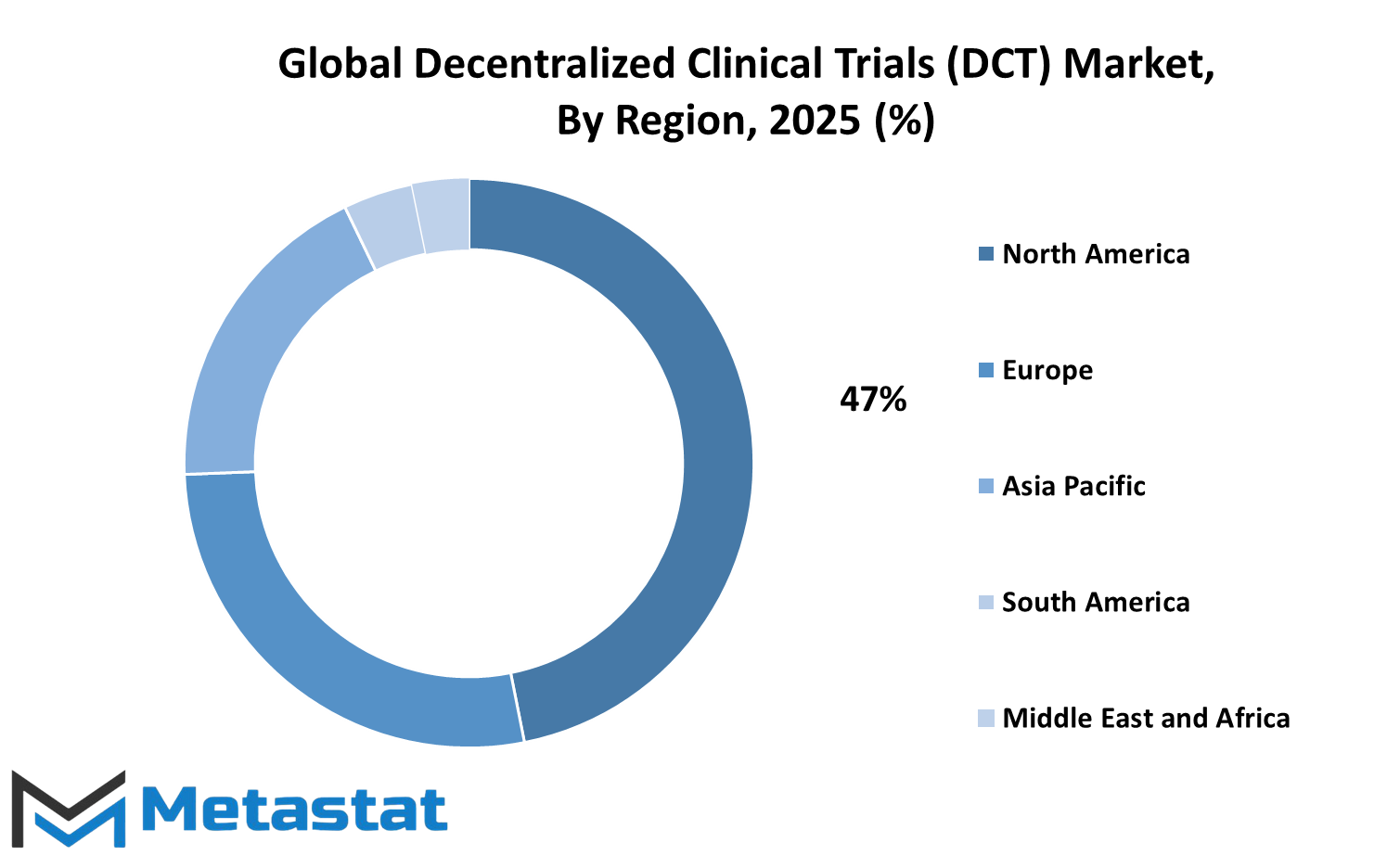
The global decentralized clinical trials (DCT) market is gradually determining the fate of medical trials, and one can experience its development across various regions on the globe. With the health care industry more and more hospitable to online processes, utilization of decentralized procedures will surely continue to grow in small increments. These advancements are not taking place at an equal pace everywhere because each region has its strengths and weaknesses that dictate the pace with which this model is adopted and utilized. North America will remain at the forefront of this shift, mainly because of its strong research culture and early adoption of digital instruments.
The United States, in particular, will remain at the forefront of this. Its large network of pharmaceutical companies and research communities translates to faster experimentation and application of decentralized methods. Canada and Mexico are also gaining momentum in the field, showing interest in hosting remote trials to provide healthcare opportunities to inhabitants of rural places far from medical facilities. Europe also stands to be a leading force. Countries like the UK, Germany, France, and Italy are focusing increasingly on patient-friendliness in clinical trials.
They are building stronger digital networks and encouraging companies to embrace virtual tools in trial processes. While regulatory variations between countries can cause some delays, the overall trend towards remote trials is sure to keep gathering pace. European governments and organizations are starting to come together more to increase the efficiency of these trials and make them more reliable for patients. Decentralized trials are experiencing growing interest in Asia-Pacific. Big populations and growing access to mobile technologies in countries such as India, China, Japan, and South Korea position them well to take up such strategies.
Every one of these poses unique challenges, e.g., divergent health standards and connectivity to technology for urban and rural areas, but the potential is great. The growing number of patients and sustained investment in the healthcare infrastructure will fuel this market. South America and Middle East & Africa remain in the infancy stages, yet they are well on their way. Brazil and Argentina, and even countries such as South Africa and the ones in the GCC, are beginning to try out decentralized models. These take a bit longer to build up, but interest is gathering pace, especially where traditional clinical trials have been limited. The global decentralized clinical trials (DCT) market will certainly increase as every one of these nations finds its own way of adapting and profiting from distance healthcare trials.
COMPETITIVE PLAYERS
The global decentralized clinical trials (DCT) market is slowly shaping the future of how medical studies are conducted, offering new opportunities for both researchers and participants. With technology becoming more accessible and dependable, this method of clinical research is gaining momentum. It allows participants to be involved without having to visit trial sites in person, which not only helps reduce travel and wait times but also improves access for people who may not live near research centers. As more healthcare providers and pharmaceutical companies shift toward this model, several key players have emerged who are helping to shape and support this growth.
Companies such as IQVIA, Parexel, ICON, and Medidata Solutions are pushing boundaries by creating tools and platforms that allow for smooth data collection and communication between patients and researchers. Labcorp and Oracle are also working on digital systems that will make patient monitoring and data storage more secure and easier to manage. These efforts are important because they help reduce delays in trials and improve data accuracy, both of which are crucial in medical research. Clinical Ink and Medable are also contributing with software that simplifies remote patient tracking, helping researchers stay connected with participants in real time.
What sets these organizations apart is their focus on combining technology with patient care. By using apps, sensors, and wearable devices, they are helping to bring trials directly to people’s homes. This approach not only makes the process more convenient but also improves participation rates, especially among those who might otherwise face barriers. Companies like Science 37 and PPD, now a part of Thermo Fisher Scientific, are focusing on building networks that make these virtual trials possible on a global scale. This worldwide reach could lead to more diverse patient pools and stronger results.
Other important names, including Huma, Signant Health, and Syneos Health, are investing in personalized experiences for trial participants, while groups like Advarra and Curavit Clinical Research Corporation are focusing on compliance and safety, ensuring all guidelines are followed. Medpace is also playing a major role by supporting end-to-end services for digital trials. Looking ahead, these companies are expected to keep leading innovations in this space.
As technology grows and becomes more a part of everyday healthcare, the global decentralized clinical trials (DCT) market will likely become a standard part of how medical research is done around the world, helping reach people where they are and speeding up access to new treatments.
Decentralized Clinical Trials (DCT) Market Key Segments:
By Study Type
- Interventional
- Observational
- Expanded Access
By Component
- Wearable Devices
- eConsent Solutions
- Telemedicine Platforms
- Data Management Software
By Application
- Oncology
- Cardiology
- Neurology
- Respiratory
By End-User
- Pharmaceutical Companies
- Biotechnology Firms
- Contract Research Organizations (CROs)
- Academic and Research Institutions
Key Global Decentralized Clinical Trials (DCT) Industry Players
- IQVIA
- Parexel
- ICON
- Medidata Solutions
- Labcorp
- Oracle
- Clinical Ink
- Medable
- Science 37
- PPD (Part of Thermo Fisher Scientific)
- Huma
- Signant Health
- Syneos Health
- Advarra
- Curavit Clinical Research Corporation
- Medpace
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential




 US: +1-(714)-364-8383
US: +1-(714)-364-8383






