MARKET OVERVIEW
The global contract research organization (CRO) market is a key component of the pharmaceutical and biotechnology sector, and it plays a fundamental role in facilitating drug development, clinical trials, and regulatory affairs. The market will be fueled by increasing demand for outsourced capabilities and cost-saving measures across all phases of research and development. The Global CRO market will address firms that seek to improve their operations by outsourcing complicated operations like clinical trial management, biostatistics, pharmacovigilance, and medical writing to organizations with scientific capabilities and operational agility. The market will span across a broad spectrum of services from early discovery stages to late-stage clinical trials.
Service providers will provide more than technical strengths but also enable regulatory strategy, as well as global site management. Strategic partnering between CROs and sponsors will define the global contract research organization (CRO) market, recognizing life sciences firms to remain agile when growing in emerging regions without making commitments in infrastructure. The partnerships will act as a platform for innovation, while ensuring operational burdens remain light for sponsoring firms. As pharmaceutical pipelines become more complicated and regulatory needs tighter, the global contract research organization (CRO) market will act as a link between scientific innovation and compliance needs. Compliance requirements in regions will call for region-specific strategies, and CROs will bridge the gap by providing region-specific solutions and flexible delivery models.
With this professional expertise, the pharmaceutical and biotech industries will be able to launch their products more effectively on a global scale. The geography of the global contract research organization (CRO) market will cover a variety of therapeutic indications such as oncology, cardiology, neurology, infectious diseases, and metabolic disorders. All these therapeutic indications would involve different clinical protocols, patient recruitment processes, and data analysis strategies. CROs will keep expanding by spending on data technologies, artificial intelligence solutions, and patient-focused platforms, aligning their capabilities to emerging demands confronting clinical trials and measuring health outcomes.
Work in biosimilars, advanced therapies, and orphan drugs, with complex trial designs and regulatory approaches, will also be funded by the market. Geographically, global contract research organization (CRO) market will find activity from Asia-Pacific, Latin America, North America, and Europe, each contributing according to individual regulatory landscapes and cost parsimony. Local monitoring and activity of the CROs will be equipped for aiding trial deployment across borders, while adhering to national regulation and scientific integrity. The future of CRO market dynamics will be determined not only by scientific breakthroughs but, to a larger extent, by evolving service delivery models, consolidation among providers, and increasing emphasis on patient diversity and transparency. While the pharmaceutical and biotech communities respond to the pace and direction of technological innovation, regulatory changes, and shifting patient expectations, CROs will be well-placed to be agile partners with the ability to break through operational and scientific barriers. Together, the global contract research organization (CRO) market will be a pillar in the architecture of drug development globally to enable greater and scientifically more rational developments throughout the horizon of healthcare.
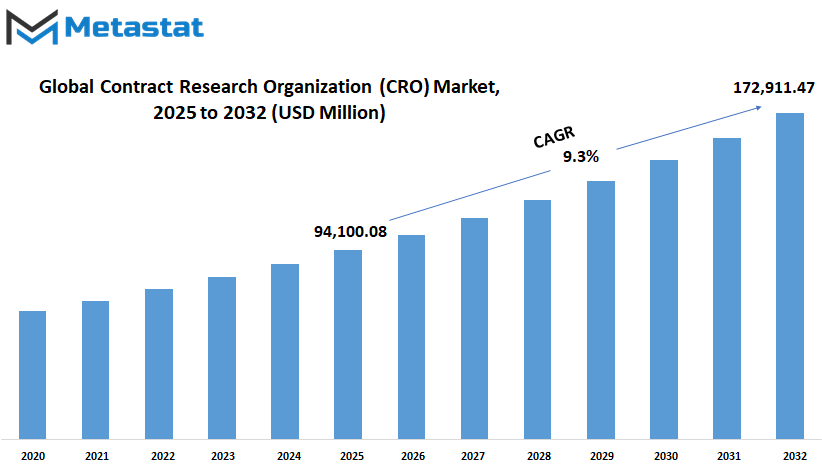
Global contract research organization (CRO) market is estimated to reach $172,911.47 Million by 2032; growing at a CAGR of 9.3% from 2025 to 2032.
GROWTH FACTORS
The global contract research organization (CRO) market is set to experience phenomenal growth over the next two years or so, courtesy of some of the strongest driving forces that pharma and biotech companies experience in undertaking their research. The key reason for this upsurge is the constant increase in research and development spending. Pharmaceutical organizations are increasingly investing in research to create new drugs and medicine, and they need partners who can speed up the process. With the assistance of contract research organizations, pharmaceutical firms can access specialized expertise and infrastructure to bring new medicine to the market faster than would be achievable with it all retained in-house. The same strong driving force for the market also lies in the increasing trend of outsourcing by pharmaceutical companies.
Since it is a time and cost-consuming process to take a new drug from the lab to the patient, reliance on CROs allows the pharma companies to keep their core areas of expertise such as drug development and marketing and subcontract the technical and generally costly clinical trials to the experts. Not only does this division of labor enhance efficiency but also the overall risk of drug development projects is lowered. In spite of these positive trends, the Global Contract Research Organization market also has some drawbacks. The regulatory requirements are increasing and the clinical trials approval process is slow and uncertain. These delays can delay the development and cause headaches to customers' and CROs' planning.
With outside use of sensitive patient information, there is also the risk of data compromise or misuse, potentially compromising trust and creating legal problems. In the days ahead, the market should be supported by growth in novel clinical trial models like decentralized and virtual trials. Such strategies leverage technology to help patients participate in studies through distant locations with minimal need to travel to clinical sites over and over again. This shift not only makes the trials convenient and easily accessible but also expedites the research process together with cost reduction.
As more firms adopt such new trial designs, CROs that are capable of providing expertise in such areas will have ample opportunities for expansion and success. Overall, the global contract research organization (CRO) market is in a solid growth pattern based on growing R&D spending as well as the necessity for accelerated drug development through outsourcing. Although regulatory issues and data protection concerns continue to exist, the creation of decentralized and virtual trials offers new promise to the industry. This mix of factors will dictate the path of the market over the coming years, setting an environment where CROs will increasingly have a leading role in getting new drugs to patients globally.
MARKET SEGMENTATION
By Type
The global contract research organization (CRO) market shall be a prime mover in redefining the future of the pharmaceutical and biotechnology industries. CROs play crucial back-end roles for companies by undertaking all levels of research and development so that pharmaceutical manufacturers can concentrate on core business. One of the primary ways the global contract research organization (CRO) market is structured is by the nature of the service it performs, which includes drug discovery, pre-clinical studies, and clinical testing. All these segments will prosper and expand as technology continues to improve and the need for successful drug development expands. Drug discovery is the initial step in creating new drugs.
It encompasses selecting possible compounds that can become possible drugs. The global contract research organization (CRO) market will be at the mercy of innovations enhancing the accuracy and speed of this process through such processes as artificial intelligence and sophisticated data analysis. That will assist in making it shorter to come up with potential candidates as well as for better success rates. Companies in this sector will continue to collaborate with pharmaceutical firms to offer skills and access to resources that may not be internally available. After drug discovery, pre-clinical research entails the testing of these candidates in laboratory and animal testing to evaluate their safety and biological activity. This phase is essential before starting human trials.
The global contract research organization (CRO) market will evolve by delivering more niche services here, for instance, advanced testing methods and models which more accurately project the human response. As the regulations tighten, CROs will have to ensure that their studies are of high quality as well as efficient. This will enable faster transition from pre-clinical to clinical phases. Clinical trials are the most transparent aspect of drug development and entail the testing of new treatments among human volunteers to determine their safety and efficacy. The world Contract Research Organization (CRO) industry will grow in this segment with increasingly more trials being undertaken around the globe, particularly in areas that have developing healthcare infrastructure. CROs will leverage new technologies to make more efficiently the recruitment of patients, capturing data, and monitoring processes, hence making trials more faster and reliable.
In the future, genetic information and customized medicine will have an effect on the design of clinical trials, and CROs will have a vital part in managing such advanced studies. Overall, the global contract research organization (CRO) market will be an even more important ally for drug makers. With focus on drug development, pre-clinical development, and clinical trials, CROs will bring new drugs to patients in a better way and in a more secure way. The market will expand further by optimizing best use of innovation and according to evolving healthcare industry needs.
By Service Phases
The global contract research organization (CRO) market is increasing gradually and will continue to do so in the next few years. CROs are a big help for pharmaceutical and biotech firms to conduct clinical trials more effectively. CROs offer services at various phases of clinical research, simplifying the entire process of drug development and making it cost-saving. Knowledge of the different stages of services in the global contract research organization (CRO) market provides insight into how the industry plays a role in bringing new treatments from the lab to the patient. The initial clinical trial stage is a test of the new treatment or medicine on a small number of healthy patients or volunteers.
This stage is significant because it concerns safety and how the body reacts to the treatment. The global contract research organization (CRO) market helps here by providing skills and resources to undertake these initial trials. CROs make sure that the trials are carried out in accordance with very strict guidelines and help with collating quality information. Technology and data management systems employed at this stage will become more advanced as the market matures, thereby ensuring faster and efficient outcomes. Subsequently, the second stage carries out the trials to more patients to evaluate the efficacy of the treatment and to continuously monitor for safety. The function of the global contract research organization (CRO) market now is to handle more complex trial designs and to co-ordinate across multiple locations or sites.
The future advancements in this stage will likely include better patient recruitment strategies and more efficient monitoring processes, which will minimize delays and enhance the quality of information accumulated. This stage typically determines if the treatment would be continued or not, and hence the contribution of CROs is of critical importance. The third stage comprises major trials to verify the treatment's efficacy, observe side effects, and benchmark with current therapies. During this phase, the global contract research organization (CRO) market will process colossal amounts of information and ensure strict regulatory compliance. The application of artificial intelligence and big data analysis in the future will enable CROs to control such high-volume trials more efficiently, reducing the time hopefully to approve new medicines. Last but not least, the fourth stage comes when the treatment has passed through approval and is introduced in the public. The stage tracks the long-term impact and safety in a large population.
The global contract research organization (CRO) market will also continue to have a significant role to play by gathering real-world data and aiding companies in knowing how their treatments turn out post-controlled trials.
With growing health care institutions, it will be business as usual to integrate real-time data collection tools into systems in order to respond faster to any safety issue. Overall, the future for the global contract research organization (CRO) market is bright. Technological innovation and increased demand for effective drug development mean that CROs will become increasingly the lifeline partners in clinical research. Through their capacity to design and enhance every step of service, they will provide more effective treatment to patients globally, earlier and with more assurance.
By Therapeutic Areas
The global contract research organization (CRO) market a very important contribution to provide towards facilitating the development of new medical therapies in the way of offering study services to drug as well as bio-tech firms. With medical science advancing, there will be an increasing demand for specialized research firms who can perform clinical trials as well as other studies. They facilitate the delivery of new drugs, and therapies to patients more effectively by handling different stages of research and development, and the market for such services will continue to grow as more firms seek partnerships to save costs. When considering the global contract research organization (CRO) market segmented on the basis of therapeutic areas, areas of medicine are diligently and in demand divergently.
Oncology, the analysis and treatment of cancer, is one of the largest and strongest areas. Cancer is a global health issue, and there will always be a lack of trying to find improved treatments and cures. Therefore, CROs with oncology experience will have more opportunities as drug firms run long and in-depth trials to try to research new cancer therapies. Cardiology, which is a sickness of heart disease, is yet another interesting article. Heart disease still afflicts a large segment of the populace, and developments in treatment will fuel demand for research. CROs having a presence there will facilitate clinical trials for emerging drugs and equipment to treat heart disease.
Their skills will be instrumental in identifying safety and effectiveness before these products find their way into patients' hands. Neurology, the study of nervous system diseases, provides room for growth in the Global Contract Research Organization (CRO) sector. Alzheimer's, Parkinson's, and multiple sclerosis are diseases that need to be researched continuously to determine treatments that will halt or slow them down. The complexity of these diseases ensures that CROs that specialize in neurology will be performing detailed, in-depth studies to assess possible treatments.
In the case of infectious diseases, they are a changing environment and ongoing part of the marketplace. New threats on the global scale have pointed to the necessity for speedy vaccine and treatment development of viral and bacterial infections. Infectious disease CROs will be playing a critical role in running trials to verify the safety and efficacy of medicines and vaccines for public use.
Finally, metabolic disorders such as diabetes and obesity will be fueling demand in this sector too. These conditions are on the rise among increasingly more individuals throughout the world, which is spurring research to improve handling and cure. CROs operating in this segment will aid in testing new therapies for improving the quality of life in metabolic patients.
The global contract research organization (CRO) market in the future will be influenced by medical science progress and growing demand for high-quality research services. Across therapeutic areas, demand will grow as companies focus on creating new treatments. In providing clinical trials and research support, CROs will still be integral to healthcare in the future.
By End-Users
The global contract research organization (CRO) market has the potential to become an even greater player in healthcare and science research in the years to come. As demand for effective and niche research solutions increases, each of the different end-users in the market will be increasingly dependent upon CROs to power their operations. Of these clients, pharma companies will continue to be one of the largest customers because they rely on CROs to conduct clinical trials and regulatory activities. They will seek to accelerate drug development in a safe, compliant manner, and CROs will have the specialized skill set available to accomplish these objectives. Biotech companies will also increasingly rely on CROs for assistance in gene research, cell therapy, and personalized medicine.
As new technologies evolve, these companies will need highly skilled research collaborators who will be able to make those changes the new scientific requirements demand. CROs will provide flexibility and access to skilled professionals and advanced technology for biotech companies to drive their innovations from the laboratory to the marketplace more effectively. This partnership will continue to strengthen as the biotech industry develops and introduces new treatment technologies. Medical device companies will increasingly go to CROs for help in cutting through difficult testing and approval protocols. The testing for safety and efficacy of new devices is a demanding process, and CROs will be the prime sources of information and assets to get these things done. As more medical technology is developed, the partnership between device companies and CROs will become even more vital to ensure performance and deliver new devices to patients faster. Academic and research institutions will also gain from the services of the global contract research organization (CRO) market through external access to resources and technical expertise that is not internal.
Basic science and early-stage research are typically conducted by educational and research institutes, and collaboration with CROs will enable them to enhance their potential, speed up projects, and concentrate on innovation. As research needs are more sophisticated and cross-disciplinary in nature, CROs as drivers of scholarly activity will play an even greater role, filling gaps from discovery to everyday use. In the years to come, the global contract research organization (CRO) market will be further integrated into the research and development functions of its end-users. The trend will shift towards increased collaboration, with CROs not only offering services but strategic partnerships that will enable firms and institutions to better and more efficiently execute their work. Such collaboration would be crucial in the face of future healthcare demand challenges and scientific breakthroughs.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$94,100.08 million |
|
Market Size by 2032 |
$172,911.47 Million |
|
Growth Rate from 2025 to 2032 |
9.3% |
|
Base Year |
2024 |
|
Regions Covered |
North America, Europe, Asia-Pacific, South America, Middle East & Africa |
REGIONAL ANALYSIS
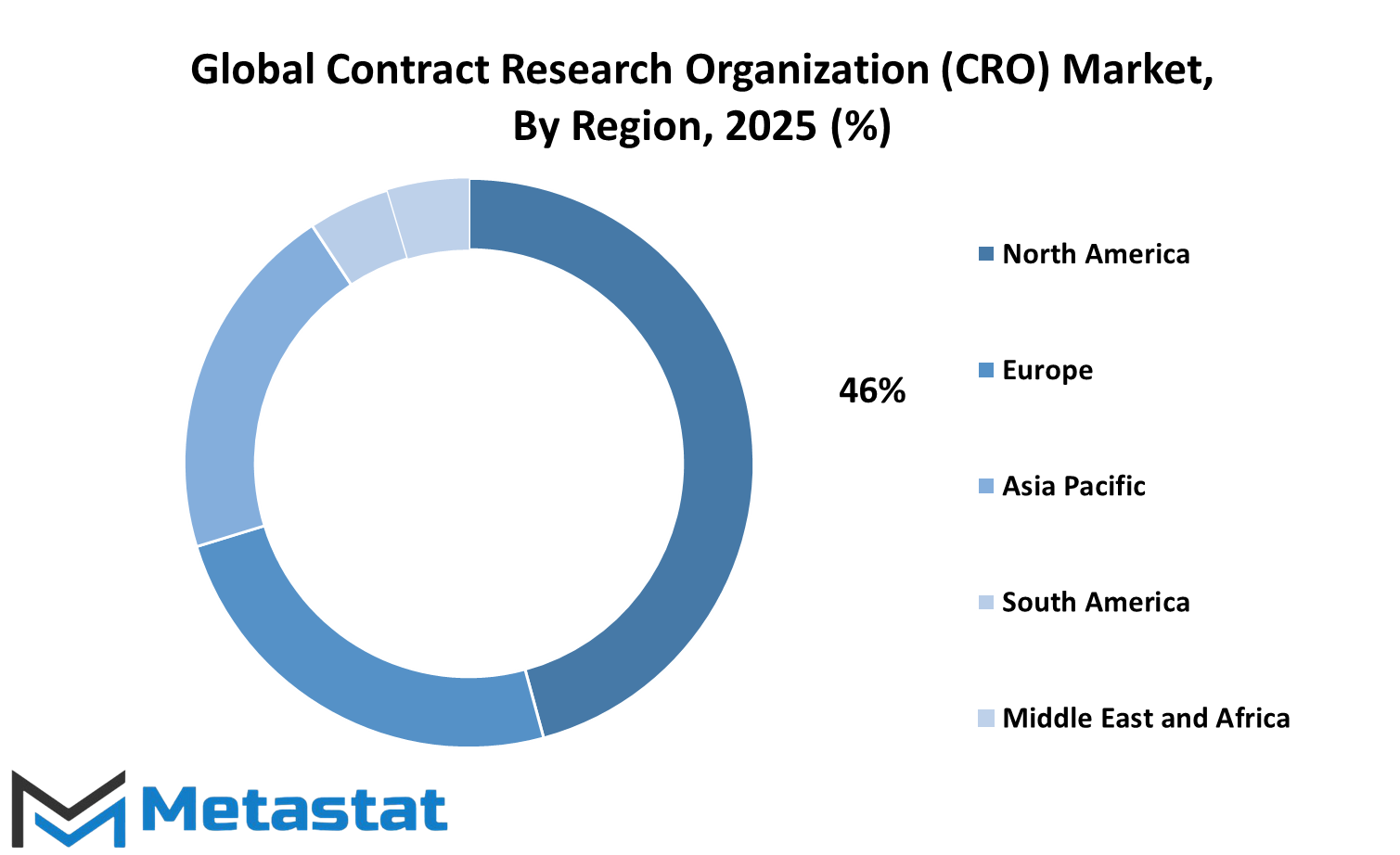
The global contract research organization (CRO) market is poised to witness significant growth in various geographies of the world. The market is being influenced by several regional trends that continue to shape the nature of clinical trials, research work, and pharmaceutical partnerships. In terms of the geographical segmentation, every region has its own strengths and weaknesses, presenting a dynamic environment for both new and mature CROs.
North America is favorably positioned with the availability of sophisticated healthcare infrastructure and huge investment in research and development. The United States, more specifically, is influential because of its numerous pharma companies and a well-defined regulatory system. Mexico and Canada, though smaller in size, are more and more becoming a part of the rising trend of offshoring clinical trials, due to enhanced research capabilities and cost advantages. In the next few years, North America will continue to lead but will also experience rising competition.
Europe also indicates a strong presence in the global contract research organization (CRO) market. Germany, the UK, France, and Italy are among the countries with a long history of scientific research and pharmaceutical innovation. These countries continue to be preferred for clinical studies through high-quality patient care and data. Meanwhile, the Rest of Europe is slowly catching up with increasing investment by governments and companies in life sciences and technology. While regulatory procedures in Europe are rigorous, they are usually regarded as transparent and reliable, and hence the continent is a favorite of most international sponsors.
Asia-Pacific is also quickly emerging as a force. Countries like China, India, Japan, and South Korea are coming into the limelight for having huge patient pools, economies of scale, and advancements in clinical trial infrastructure. India and China, in particular, will be at the epicenter of this market's future. Digital revolution, friendly policies, and local talent are leading this region ahead. With the growth of CROs in these nations, the general equilibrium of the global contract research organization (CRO) market is gradually moving eastward.
Middle East & Africa and South America are also expanding, albeit more slowly. South American nations like Brazil and Argentina are strengthening their networks of research, while Egypt, GCC nations, and South Africa are busy increasing their attractions by introducing healthcare reforms and partnerships with the global world. These markets are gradually going to receive more attention over time, particularly as they look to untapped markets and heterogeneous populations of patients. With increasing healthcare demands and developing technology, all regions will contribute towards the direction of this international market.
COMPETITIVE PLAYERS
The global contract research organization (CRO) market is increasingly in the spotlight as the pharmaceutical and healthcare sectors increasingly rely on external assistance for clinical development and research services. Over time, the CROs have increasingly become pivotal to how new medicines are tested and brought to the masses. They help pharmaceutical, biotech, and medical device firms oversee clinical trials, meet regulatory requirements, and gather data that will drive the future of medicine. With increasing demand for faster and more effective trials, these groups will have even greater responsibilities to assume. In the years ahead, their existence will not only aid in the evolution of new treatments but also determine how healthcare systems react to patient needs.
The global contract research organization (CRO) market is so topical today is that the way businesses conduct research has changed. Most companies now turn to outside of their internal staff and use CROs to oversee studies with a degree of intensity and scope that would otherwise be unmanageable. This is not merely a cost-cutting exercise but gaining access to certain skills that CROs provide. These companies assemble experienced staff and established methodologies that assist in streamlining trials and minimizing delays. Over the near term, they will probably have an increasingly important role in responding to new technologies such as artificial intelligence and machine learning, which have the ability to determine trends and results more quickly than ever before.
There are a number of key players who have already positioned themselves in this field and will probably dominate going forward as innovation expands. Organizations such as IQVIA, Parexel, ICON, and Syneos Health have reputations for working with large and complex studies. Others, such as Lindus Health, Medpace, and CTI Clinical Trial and Consulting Services, are gaining prominence with more adaptable methods and faster turnaround. These providers provide various strengths, but all strive for the same mission making medical study quicker, safer, and more efficient.
As worldwide medical concerns continue to complicate, the assistance CROs provide will be increasingly in demand. There is obvious movement toward outsourcing in this area, and with it, new prospects for expansion and evolution. The global contract research organization (CRO) marketplace will not only grow but revolutionize how medical research is provided. With continual improvements and increasing demand for customized solutions, CROs will be at the forefront of enabling life science firms to bring meaningful treatments to the people that need them the most.
Contract Research Organization (CRO) Market Key Segments:
By Type
- Drug Discovery
- Pre-Clinical
- Clinical
By Service Phases
- Phase I Trials
- Phase II Trials
- Phase III Trials
- Phase IV Trials
By Therapeutic Areas
- Oncology
- Cardiology
- Neurology
- Infectious Diseases
- Metabolic Disorders
By End-Users
- Pharmaceutical Companies
- Biotechnology Firms
- Medical Device Manufacturers
- Academic and Research Institutions
Key Global Contract Research Organization (CRO) Market Players
- IQVIA
- Parexel
- Lindus Health
- ICON
- Syneos Health
- PPD
- Fisher Clinical Services
- KCR
- Medpace
- Worldwide Clinical Trials
- CTI Clinical Trial and Consulting Services
- WuXi AppTec
- Advanced Clinical
- Pharm-Olam
- Charles River Laboratories
- PSI CRO
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential




 US: +1 3023308252
US: +1 3023308252






