MARKET OVERVIEW
In the dynamic landscape of biopharmaceuticals, the Global Continuous Bioprocessing market emerges as a transformative force, reshaping the industry's operational paradigms. This sector represents a fusion of innovative technologies and methodologies, revolutionizing the production processes of biopharmaceuticals. Unlike conventional batch processing methods, continuous bioprocessing operates seamlessly, with uninterrupted flow, enhancing efficiency and productivity while reducing costs and time constraints.
The Global Continuous Bioprocessing market lies a spectrum of advanced tools and techniques designed to streamline biopharmaceutical manufacturing. From continuous cell culture systems to integrated purification platforms, this market epitomizes the convergence of cutting-edge engineering and bioprocess expertise. The adoption of single-use technologies further amplifies the flexibility and scalability of continuous bioprocessing, enabling manufacturers to adapt swiftly to evolving market demands.
In the foreseeable future, the Global Continuous Bioprocessing market is poised to witness exponential growth, driven by a multitude of factors. As the industry continues to prioritize agility and cost-effectiveness, the demand for continuous bioprocessing solutions will surge, propelling market expansion across various regions. Moreover, the escalating complexities associated with traditional batch processing methods will incentivize biopharmaceutical companies to embrace continuous manufacturing as a sustainable alternative.
The advent of next-generation biologics and personalized medicine heralds a new era of opportunities for the Global Continuous Bioprocessing market. With an increasing emphasis on precision and quality in biopharmaceutical production, continuous bioprocessing emerges as a catalyst for innovation, enabling manufacturers to achieve unprecedented levels of product consistency and purity. Furthermore, the integration of advanced analytics and artificial intelligence into continuous bioprocessing workflows holds the promise of enhancing process optimization and predictive maintenance, further augmenting the market's growth trajectory.
As regulatory agencies worldwide acknowledge the potential benefits of continuous bioprocessing, the industry is poised to witness a paradigm shift in manufacturing standards and compliance requirements. The establishment of regulatory frameworks tailored to continuous manufacturing practices will instill confidence among stakeholders and accelerate market adoption. Additionally, collaborations between industry players and academic institutions will foster the development of novel technologies and methodologies, driving continuous innovation within the Global Continuous Bioprocessing market.
The Global Continuous Bioprocessing market represents a transformative force within the biopharmaceutical industry, revolutionizing manufacturing processes and redefining operational standards. With its inherent advantages in efficiency, flexibility, and quality, continuous bioprocessing is poised to reshape the landscape of biopharmaceutical production in the years to come. As the industry continues to evolve and embrace innovation, the Global Continuous Bioprocessing market will remain at the forefront of driving progress and shaping the future of biomanufacturing.
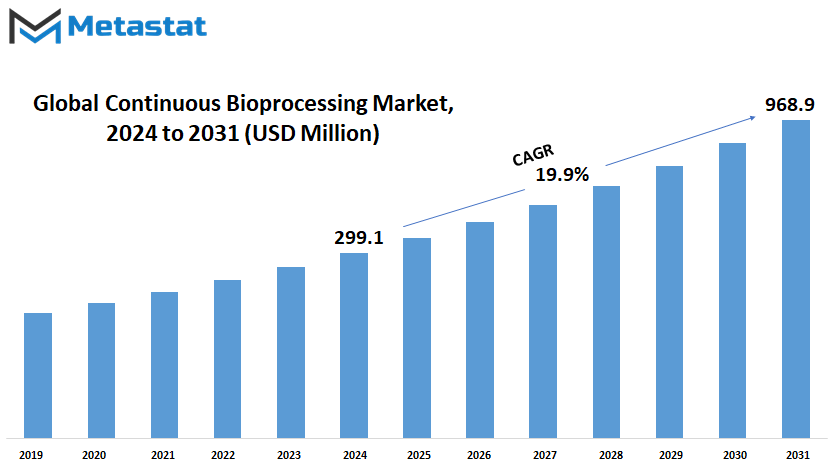
Global Continuous Bioprocessing market is estimated to reach $968.9 Million by 2031; growing at a CAGR of 19.9% from 2024 to 2031.
GROWTH FACTORS
The Global Continuous Bioprocessing market is on the rise, largely due to the increasing demand for biopharmaceuticals, which has been fueled by the growing prevalence of chronic diseases. This demand has led to a shift towards continuous bioprocessing methods, which offer enhanced efficiency and productivity compared to traditional batch processing techniques.
One of the primary drivers of this market is the need for more efficient production methods in the pharmaceutical industry. Continuous bioprocessing allows for a more streamlined production process, reducing the time and resources required to manufacture biopharmaceuticals. This increased efficiency translates to cost savings for pharmaceutical companies, making continuous bioprocessing an attractive option for production
However, there are challenges that need to be addressed in order for the market to reach its full potential. One such challenge is the high initial investment costs associated with setting up continuous bioprocessing facilities. These costs can be prohibitive for some companies, particularly smaller players in the market. Additionally, there are regulatory challenges that need to be navigated, including the need for validation of continuous manufacturing processes. Ensuring compliance with regulatory standards is essential for market growth, but it can also be a time-consuming and costly process.
Despite these challenges, there are promising opportunities on the horizon for the continuous bioprocessing market. One such opportunity lies in the adoption of continuous bioprocessing in emerging markets. As technology continues to advance and regulatory support for continuous manufacturing processes increases, emerging markets will become increasingly attractive for investment in continuous bioprocessing facilities. This presents an opportunity for companies to expand their market reach and tap into new sources of revenue.
The Global Continuous Bioprocessing market is poised for growth in the coming years, driven by increasing demand for biopharmaceuticals and the efficiency gains offered by continuous bioprocessing methods. While there are challenges that need to be addressed, such as high initial investment costs and regulatory hurdles, there are also significant opportunities for market expansion, particularly in emerging markets. With the right investments and strategic planning, the continuous bioprocessing market will continue to thrive in the future.
MARKET SEGMENTATION
By Product
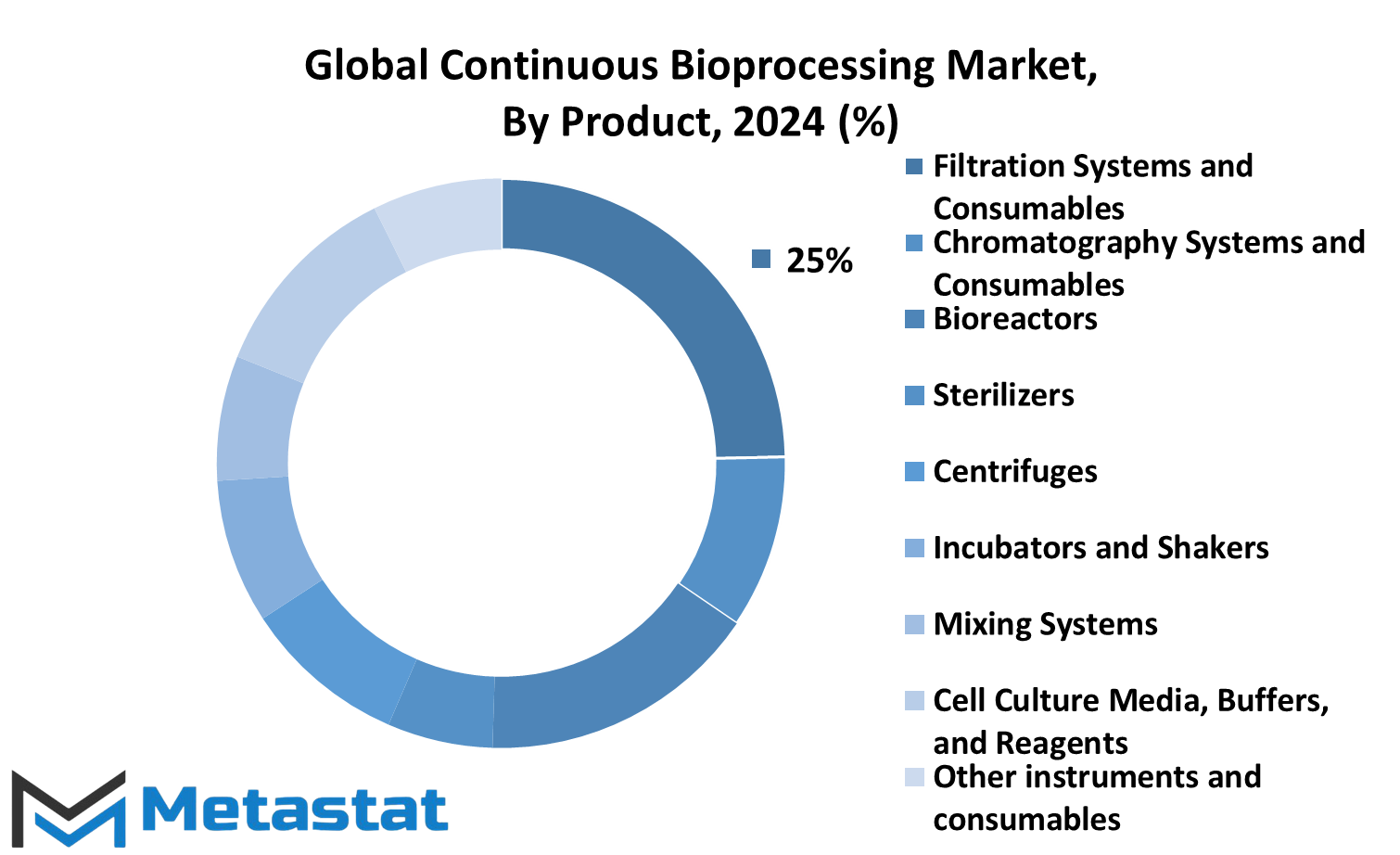
The Global Continuous Bioprocessing market is witnessing notable growth, propelled by various factors such as technological advancements, increasing demand for biopharmaceuticals, and the need for efficient production processes. Within this market, products are categorized into several segments, including Filtration Systems and Consumables, Chromatography Systems and Consumables, Bioreactors, Sterilizers, Centrifuges, Incubators and Shakers, Mixing Systems, Cell Culture Media, Buffers, and Reagents, as well as other instruments and consumables.
Continuous Bioprocessing is gaining traction due to its advantages over traditional batch processing methods. It offers increased productivity, reduced production time, and enhanced product quality. This approach enables manufacturers to achieve higher yields and better control over the manufacturing process.
Filtration Systems and Consumables play a crucial role in bioprocessing by removing impurities and particulates from the product stream. They ensure the purity and integrity of the final product. Chromatography Systems and Consumables are utilized for separation and purification processes, allowing for the isolation of specific biomolecules.
Bioreactors are essential equipment used for the cultivation of cells and microorganisms in a controlled environment. They provide the necessary conditions for cell growth and expression of desired products. Sterilizers are employed to maintain a sterile environment within the bioprocessing facility, preventing contamination of the product.
Centrifuges are utilized for the separation of components based on their density, enabling the isolation of target molecules. Incubators and Shakers are used for the cultivation of cells under optimal conditions, facilitating their growth and proliferation.
Mixing Systems play a vital role in bioprocessing by ensuring homogeneity and uniformity of the product stream. They facilitate the mixing of various components and solutions, contributing to the efficiency of the manufacturing process.
Cell Culture Media, Buffers, and Reagents are essential components used in bioprocessing for cell culture maintenance, pH regulation, and other biochemical processes. They provide the necessary nutrients and support for cell growth and viability.
Other instruments and consumables encompass a wide range of equipment and materials used in bioprocessing, including sensors, probes, and disposable bioreactors. These tools contribute to the overall efficiency and productivity of the manufacturing process.
The Global Continuous Bioprocessing market is witnessing significant growth, driven by advancements in technology and increasing demand for biopharmaceuticals. The segmentation of products into various categories reflects the diverse range of equipment and consumables utilized in bioprocessing operations. As the industry continues to evolve, continuous innovation and development will play a key role in shaping the future of bioprocessing.
By Process Type
The Global Continuous Bioprocessing market is segmented based on process type into Upstream Processes and Downstream Processes.
Upstream processes involve the cultivation of cells and their growth in bioreactors. These processes are crucial as they lay the foundation for the production of biopharmaceuticals. Continuous bioprocessing in upstream processes will enable a constant supply of cells, ensuring a steady production flow. This will enhance efficiency and productivity, as there will be no need for batch-to-batch adjustments, leading to cost savings and reduced time-to-market for biopharmaceutical products.
Downstream processes, on the other hand, involve the purification and isolation of the desired biopharmaceutical product from the cell culture. Continuous bioprocessing in downstream processes will streamline purification steps, resulting in higher product purity and yield. This will be achieved through the integration of various unit operations, such as chromatography and filtration, into a single continuous process. As a result, there will be fewer manual interventions and reduced risk of product contamination, thereby ensuring product quality and safety.
The adoption of continuous bioprocessing in the biopharmaceutical industry will also facilitate real-time monitoring and control of bioprocess parameters. Advanced sensors and analytics will enable continuous monitoring of critical process parameters, such as cell viability and product concentration. This will provide greater insights into process dynamics and enable timely interventions to optimize process performance. Moreover, automation and artificial intelligence will play a significant role in optimizing process parameters and ensuring process robustness.
By Application
The Global Continuous Bioprocessing market is a crucial aspect of the biotechnology sector, driving advancements in healthcare and pharmaceuticals. This market is segmented by application into Vaccines, Monoclonal Antibody (mAb) production, Recombinant Protein Production, Cell and Gene Therapy Production, and Research and Development (R&D).
Vaccines play a pivotal role in preventing infectious diseases and maintaining public health. Continuous bioprocessing offers benefits such as increased productivity, reduced production time, and enhanced product quality, making it a promising approach for vaccine manufacturing.
mAbs, a type of therapeutic protein, are widely used in treating various diseases such as cancer, autoimmune disorders, and infectious diseases. Continuous bioprocessing enables efficient production of mAbs, ensuring a stable and consistent supply to meet the growing demand for these life-saving treatments.
Recombinant proteins are essential for numerous medical and industrial applications, including diagnostics, therapeutics, and industrial enzymes. Continuous bioprocessing facilitates the scalable production of recombinant proteins with improved purity and yield, meeting the needs of diverse industries.
Cell and gene therapies represent a revolutionary approach to treating genetic disorders, cancers, and other complex diseases. Continuous bioprocessing offers advantages such as precise control over process parameters, scalability, and cost-effectiveness, supporting the development and commercialization of these advanced therapies.
Research and Development (R&D) forms the foundation of innovation in biotechnology. Continuous bioprocessing accelerates R&D activities by providing real-time data, enabling rapid process optimization, and reducing development timelines. This fosters innovation and drives the discovery of novel biopharmaceuticals and biotechnological solutions.
Moreover, increasing investments in biopharmaceutical research and manufacturing infrastructure, coupled with growing demand for personalized medicine and biologics, will fuel the expansion of the Continuous Bioprocessing market across diverse applications and regions.
The Global Continuous Bioprocessing market is poised for continuous growth and evolution, playing a vital role in advancing healthcare, pharmaceuticals, and biotechnology. By leveraging continuous bioprocessing technologies, stakeholders will drive innovation, improve patient outcomes, and address global health challenges in the years to come.
By End User
In the ever-changing landscape of bioprocessing, the global market is witnessing a significant shift towards continuous bioprocessing techniques. This evolution is driven by the demand for enhanced efficiency, reduced costs, and improved product quality in pharmaceutical and biotechnology industries, among others.
Continuous bioprocessing, unlike traditional batch processing, involves the uninterrupted flow of materials and processes throughout the production cycle. This approach offers several advantages, including higher productivity, better control over product quality, and reduced manufacturing footprint.
One of the key drivers behind the adoption of continuous bioprocessing is its ability to streamline production processes and minimize downtime. By eliminating the need for batch-to-batch transfers and cleaning procedures, continuous bioprocessing can significantly increase manufacturing efficiency and throughput.
Furthermore, continuous bioprocessing enables real-time monitoring and control of critical process parameters, allowing manufacturers to optimize production conditions and ensure consistent product quality. This level of control is particularly crucial in the pharmaceutical and biotechnology sectors, where product quality and regulatory compliance are paramount.
In addition to pharmaceutical and biotechnology companies, other end users, such as contract research organizations (CROs), contract manufacturing organizations (CMOs), and academic and research institutes, are also recognizing the potential benefits of continuous bioprocessing. These organizations are increasingly leveraging continuous bioprocessing technologies to enhance their research capabilities, accelerate product development, and improve operational efficiency.
Nevertheless, with ongoing advancements in technology and increasing industry collaboration, these challenges are expected to be overcome, paving the way for broader adoption of continuous bioprocessing solutions in the global market.
In conclusion, continuous bioprocessing represents a paradigm shift in bioprocess manufacturing, offering numerous benefits to various end users across the pharmaceutical, biotechnology, and research sectors. As technology continues to advance and industry awareness grows, continuous bioprocessing will play an increasingly integral role in shaping the future of biopharmaceutical production.
REGIONAL ANALYSIS
The global Continuous Bioprocessing market is analyzed on a regional basis, taking into account geographical distinctions. These regions include North America, Europe, Asia-Pacific, South America, and the Middle East & Africa.
In North America, which includes the United States, Canada, and Mexico, the Continuous Bioprocessing market exhibits unique trends and characteristics. Similarly, Europe, comprising the United Kingdom, Germany, France, Italy, and the rest of Europe, presents its own dynamics within the market.
Moving towards Asia-Pacific, we find a diverse landscape encompassing India, China, Japan, South Korea, and the rest of Asia-Pacific. Each of these countries contributes distinctively to the regional market scenario.
In South America, specifically in Brazil, Argentina, and the rest of South America, the Continuous Bioprocessing market showcases its particularities shaped by local factors and industry dynamics.
The Middle East & Africa region, which includes GCC Countries, Egypt, South Africa, and the rest of the Middle East & Africa, presents a unique environment for Continuous Bioprocessing market growth and development.
Each of these regions is characterized by its own set of challenges, opportunities, and regulatory frameworks, which significantly influence the trajectory of the Continuous Bioprocessing market within that area. For instance, in North America, stringent regulatory standards and a robust infrastructure for bioprocessing technology contribute to market growth. Conversely, in emerging economies within the Asia-Pacific region, factors such as increasing investments in healthcare infrastructure and rising demand for biopharmaceuticals drive market expansion.
Furthermore, regional differences in technological adoption, manufacturing capabilities, and skilled labor availability play a crucial role in shaping the competitive landscape of the Continuous Bioprocessing market. Companies operating in these regions need to navigate through these varying landscapes to capitalize on growth opportunities and establish a strong foothold in the global market.
Overall, understanding the nuances of each region is essential for stakeholders in the Continuous Bioprocessing market to formulate effective strategies, tailor products and services to local needs, and stay ahead in an increasingly competitive and dynamic global marketplace.
COMPETITIVE PLAYERS
In the fast-paced landscape of bioprocessing, the integration of continuous techniques is set to reshape the industry's dynamics. The Global Continuous Bioprocessing market is witnessing a surge in adoption, with key players such as 3M, Thermo Fisher Scientific, and Sartorius AG leading the charge. This paradigm shift is not merely a trend but a fundamental transformation poised to redefine how biologics are manufactured.
Continuous bioprocessing offers several advantages over traditional batch methods. Firstly, it enables real-time monitoring and control, ensuring consistent product quality and higher yields. Secondly, it streamlines production processes, leading to reduced manufacturing footprints and operational costs. Thirdly, it enhances scalability, allowing for seamless transition from lab-scale to commercial production.
The adoption of continuous bioprocessing is being propelled by advancements in technology and a growing demand for biopharmaceuticals. Automation, data analytics, and artificial intelligence are driving innovation, enabling tighter process control and optimization. Furthermore, the shift towards personalized medicine and the increasing complexity of biologics necessitate flexible and efficient manufacturing solutions, which continuous bioprocessing can deliver.
Key players in the continuous bioprocessing industry are investing heavily in research and development to stay ahead of the curve. Collaborations and partnerships are also on the rise, fostering knowledge exchange and accelerating innovation. Additionally, regulatory bodies are actively engaging with industry stakeholders to establish guidelines and standards for continuous manufacturing, ensuring product quality and patient safety.
Looking ahead, the continuous bioprocessing market will continue to expand as biopharmaceutical companies seek to enhance efficiency and agility in their production processes. The adoption of single-use technologies and modular manufacturing platforms will further drive growth, enabling rapid deployment and scalability. Moreover, advancements in bioprocess modeling and simulation will facilitate process optimization and predictive analytics, empowering manufacturers to make informed decisions and reduce time-to-market.
Continuous bioprocessing represents a transformative shift in the biopharmaceutical industry, offering unprecedented levels of efficiency, flexibility, and control. As technology continues to evolve and market dynamics evolve, key players will need to adapt and innovate to stay competitive in this rapidly changing landscape. With its myriad benefits and potential applications, continuous bioprocessing is poised to revolutionize how biologics are manufactured, ensuring a brighter and more sustainable future for the industry.
Continuous Bioprocessing Market Key Segments:
By Product
- Filtration Systems and Consumables
- Chromatography Systems and Consumables
- Bioreactors
- Sterilizers
- Centrifuges
- Incubators and Shakers
- Mixing Systems
- Cell Culture Media, Buffers, and Reagents
- Other instruments and consumables
By Process Type
- Upstream Processes
- Downstream Processes
By Application
- Vaccines
- mAb production
- Recombinant Protein Production
- Cell and Gene Therapy Production
- R&D
By End User
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations and Contract Manufacturing Organizations
- Academic and Research Institutes
Key Global Continuous Bioprocessing Industry Players
- 3M
- Thermo Fisher Scientific
- Sartorious AG
- Eppendorf AG
- Pall Corporation
- Cytiva
- Merck KGaA
- Repligen
- Getinge
- BIONET
- Corning Incorporated
- Fujifilm Holdings Corporation
- Entegris
- Meissner Corporation
- Eppendorf SE
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential




 US: +1 3023308252
US: +1 3023308252






