Global Cell Therapy Biomanufacturing Market - Comprehensive Data-Driven Market Analysis & Strategic Outlook
The global cell therapy biomanufacturing market will be a watershed platform in the biotechnology and healthcare sector, driving the way therapies are developed and delivered in the future. With medical science relentlessly breaking boundaries, the process of manufacturing cell-based therapies will become increasingly critical with a focus not only on innovation but on precision and safety as well. The market will go beyond its present horizon, with an accent on streamlined production processes, automation, and sophisticated quality control systems for ensuring scalability at the cost of no sacrifices in efficacy. This transformation will show a shift from conventional lab practices to highly controlled biomanufacturing environments capable of managing sophisticated therapies at a global level.
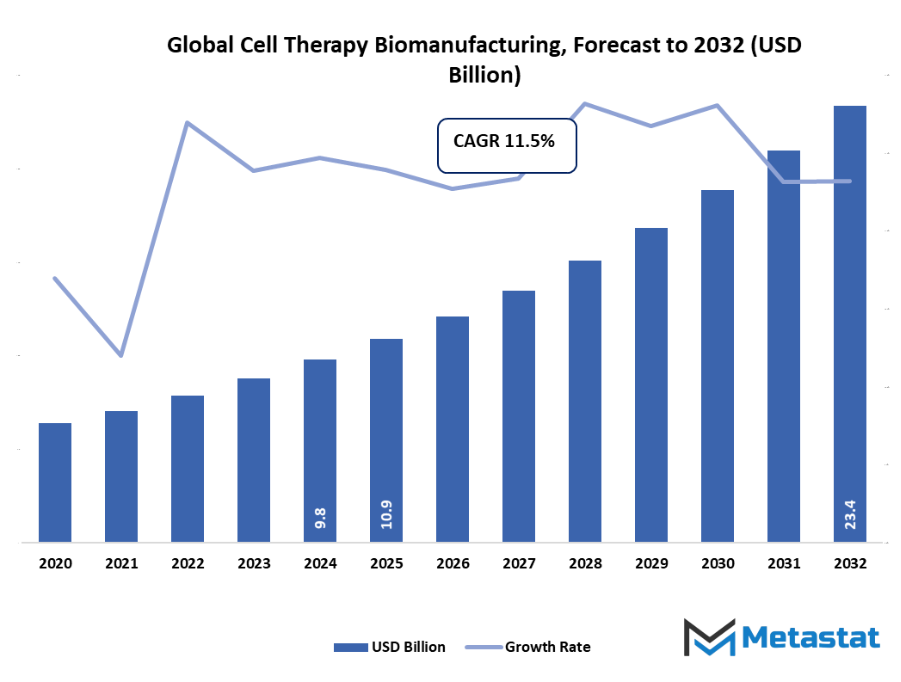
- Global cell therapy biomanufacturing market valued at approximately USD 10.9 Billion in 2025, growing at a CAGR of around 11.5% through 2032, with potential to exceed USD 23.4 Billion.
- Consumables account for nearly 55.0% market revenues, driving innovation and expanding applications through intense research.
- Key trends driving growth: Growing demand for personalized and regenerative medicine therapies., Advancements in automated and scalable cell manufacturing technologies.
- Opportunities include Expansion of contract development and manufacturing organizations (CDMOs) to support global cell therapy commercialization.
- Key insight: The market is set to grow exponentially in value over the next decade, highlighting significant growth opportunities.
Is it possible for the global cell therapy biomanufacturing market to successfully navigate the barriers of large-scale production, still retaining the effective and safe therapeutic qualities? Are the novel technologies and robots to be the ones who will take over the future of personalized medicine in this field? The question to be asked now is how the producers will harmonize cost-efficiency with the increasingly complicated regulatory norms across the globe as demand grows?
The future of the market will not be limited to treating particular diseases. Rather, it will move into territories previously considered beyond reach, coupling sophisticated genetic tools and digital tracking systems to improve speed as well as precision in manufacturing. Cell therapies are becoming more versatile, for example, through immune-cell engineering and regenerative therapies; thus, producers are already working toward new benchmarks and regulatory pathways that allow for worldwide access. Collaboration between academia, pharmaceutical companies, and tech innovators will be the main driver in determining the following phase of this market's growth.
Market Segmentation Analysis
The global cell therapy biomanufacturing market is mainly classified based on Product Type, End User.
By Product Type is further segmented into:
- Consumables: Strong demand for consumables in the global cell therapy biomanufacturing market is primarily due to more research studies and clinical trials. Besides, consumables like media, reagents, and culture vessels will be the lifeline of large-scale cell therapy production. The continuous enhancement of the formulations is driving the efficiency and safety of the cell-based products, which is the future of those products.
- Equipment: Precision and consistency will be a must in the global cell therapy biomanufacturing market and thus, sophisticated technologies will be the requirements. Automated bioreactors, centrifuges, and cell counters will be the contributing factors to the standardization of the manufacturing process. The integration of automation will reduce human errors and increase the production capacity, thereby, strengthening the global therapeutic development.
- Systems & Software : In the global cell therapy biomanufacturing market, Software and digital systems will be a game changer for production monitoring. The Intelligent data management software will be responsible for the real-time monitoring of batch records and quality parameters. On the other hand, the AI-driven platforms along with predictive analytics will give the cell growth the higher accuracy and the production will face less downtime because of that.
By End User the market is divided into:
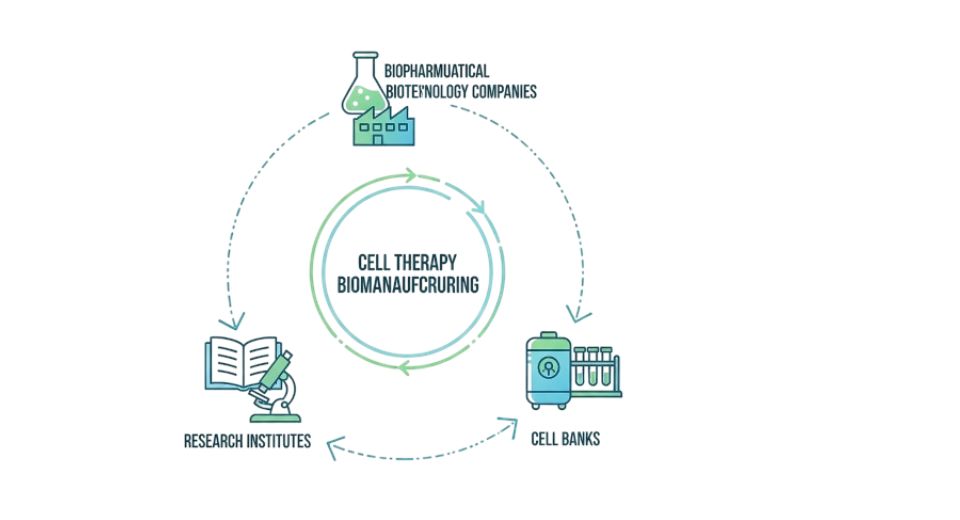
- Biopharmaceutical Biotechnology Companies: Cell therapy manufacturing technologies will be acquiring more and more from the biopharmaceutical and biotechnology companies. They will focus on cost-efficient production lines which support the preservation of cell integrity and potency. Moreover, they will be forming strategic partnerships with technology suppliers, that way they can not only accelerate the process of getting the drugs to the market but also improve the innovation.
- Research Institutes: The global cell therapy biomanufacturing market will rely heavily on research institutes for the accelerating of scientific knowledge and the refining of process designs. The growing emphasis on translational research will lead to the conversion of laboratory outcomes into medical applications. Greater investment in and expansion of infrastructure will additionally strengthen academic-industry partnerships globally.
- Cell Banks: Cell banks will serve as the essential centers for storage and supply of high-quality cell lines. The global cell therapy biomanufacturing market will be dependent on them for standardization and traceability. Investments in cryopreservation technologies and in quality assurance systems will ensure the availability of viable cell stocks for therapeutic and research applications.
- Others: The market will be further enriched by other end users like contract manufacturing organizations and hospital-based production sites. The global cell therapy biomanufacturing market will rely on versatile outsourcing schemes that allow for rapid manufacturing and alteration. The proliferation of local plants will make it possible for the world to have easier access to the most sophisticated treatments.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$10.9 Billion |
|
Market Size by 2032 |
$23.4 Billion |
|
Growth Rate from 2025 to 2032 |
11.5% |
|
Base Year |
2024 |
|
Regions Covered |
North America, Europe, Asia-Pacific, South America, Middle East & Africa |
Geographic Dynamics
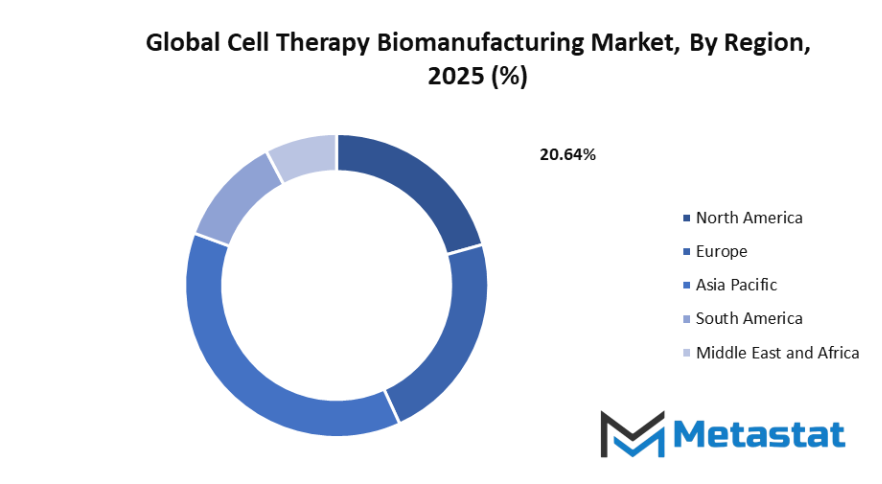
Based on geography, the global cell therapy biomanufacturing market is divided into North America, Europe, Asia-Pacific, South America, and Middle East & Africa. North America is further divided in the U.S., Canada, and Mexico, whereas Europe consists of the UK, Germany, France, Italy, and Rest of Europe. Asia-Pacific is segmented into India, China, Japan, South Korea, and Rest of Asia-Pacific. The South America region includes Brazil, Argentina, and the Rest of South America, while the Middle East & Africa is categorized into GCC Countries, Egypt, South Africa, and Rest of Middle East & Africa.
Competitive Landscape & Strategic Insights
The global cell therapy biomanufacturing market has drawn a lot of attention in the last few years due to the continuous increase in demand for advanced treatment methods. The market is dealing with the development and production of cell-based therapies that can heal or at least give significant relief to diseases previously branded as incurable. These therapies are transforming healthcare by curing diseases instead of just soothing their symptoms. The foundation for continual growth in this industry has been laid by the augmented number of clinical trials, increased research activities, and larger investments in the biotechnology field.
The industry is characterized by a combination of global leaders and newly emerging regional companies, all of whom are associated with the innovation and the efficiency of production processes. The companies such as BD, Lonza, Sartorius AG, Bio-Techne, Merck KGaA, Miltenyi Biotec, Eppendorf SE, PBS Biotech, Inc., Repligen Corporation, Corning Incorporated, Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., and Bharat Biotech International Limited are the ones that have a lot of control over market trends. These companies are known and recognized for their strong capabilities in bioprocessing, cell culture technologies, and manufacturing platforms that are capable of meeting the increasing demand for both scalable and quality therapeutic production.
The ongoing research and technological breakthroughs are changing the cell therapy manufacturing process. Through the use of robots, single-use equipment, and sophisticated bioreactors the risk of contamination is being reduced while at the same time producing more drugs. These innovations have made it possible for the companies who make the drugs to deliver them faster than ever and the patients in need of the drugs now have shorter wait times. The biotechnology companies and the hospitals are making the drug approval process and getting the drugs to the patients more geographical areas quicker.
The global cell therapy biomanufacturing market is going to grow steadily for the next few years as new therapies are moving from the lab to the clinic. The competition among the big international companies and the new small firms will lead to more innovation and the faster adoption of cost-effective production. The market will become a major driver for the future of advanced medicine along with the increasing interest from investors, tech advances in robotics and better infrastructure, thus allowing millions of patients globally to benefit from it.
Market Risks & Opportunities
Restraints & Challenges:
High cost and complexity of cell therapy production: The global cell therapy biomanufacturing market will continue to encounter financial and technical hurdles because of the high cost of cell therapy production. Production will require highly sterile environments, experts, and sophisticated bioprocessing systems, all of which drive up costs. Scaling personalized treatments will also add to the production weight.
Strict regulatory requirements and quality control challenges: As cell therapy continues to progress, regulatory guidelines are expected to get increasingly stringent which in turn will lead to longer product approval and commercialization periods. By the same token, ensuring quality and patient safety will mean that many international regulations have to be complied with. The global cell therapy biomanufacturing market will face difficulties in unifying different standards across countries, thus requiring more time and money.
Opportunities:
Ramp up CDMOs for global cell therapy commercialization: The global cell therapy biomanufacturing market will be powered by the emergence of specialized CDMOs that offer top-notch manufacturing solutions to therapy developers. These entities will provide higher scaling up, lower production cost and hastened market entry times. Strategic alliances will shape a complete infrastructure for the international cell therapy commercialization that is most optimized.
Forecast & Future Outlook
- Short-Term (1–2 Years): Recovery from COVID-19 disruptions with renewed testing demand as healthcare providers emphasize metabolic risk monitoring.
- Mid-Term (3–5 Years): Greater automation and multiplex assay adoption improve throughput and cost efficiency, increasing clinical adoption.
- Long-Term (6–10 Years): Potential integration into routine metabolic screening programs globally, supported by replacement of conventional tests with advanced biomarker panels.
Market size is forecast to rise from USD 10.9 Billion in 2025 to over USD 23.4 Billion by 2032. Cell Therapy Biomanufacturing will maintain dominance but face growing competition from emerging formats.
In addition to technological advancements, the global cell therapy biomanufacturing market will be focusing on sustainability and ethical production practices as the demand for transparency and responsible methods grows. Factories will set up new energy systems and reliable sourcing practices for their suppliers, thereby ensuring they are sustainable for the long run. In the end, the market will not only be about therapy production; it will be about changing healthcare manufacturing in a whole new way, setting the bar for how biological innovations will meet human needs in the coming decades.
Report Coverage
This research report categorizes the global cell therapy biomanufacturing market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global cell therapy biomanufacturing market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global cell therapy biomanufacturing market.
Cell Therapy Biomanufacturing Market Key Segments:
By Product Type
- Consumables
- Equipments
- Systems & Software
By End User
- Biopharmaceutical Biotechnology Companies
- Research Institutes
- Cell Banks
- Others
Key Global Cell Therapy Biomanufacturing Industry Players
- BD
- Lonza
- Sartorius AG
- Bio-Techne.
- Merck KGaA
- Miltenyi Biotec
- Eppendorf SE
- PBS Biotech, Inc.
- Repligen Corporation
- Corning Incorporated
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories, Inc.
- Bharat Biotech International Limited
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential




 US: +1 3023308252
US: +1 3023308252






