Global Aseptic Transfer Port Market - Comprehensive Data-Driven Market Analysis & Strategic Outlook
Are producers organized to adopt next-technology aseptic switch solutions as production traces push for stricter contamination manipulate and higher throughput? How will rising technology and automation reshape the reliability and safety requirements that industries have followed for years? And with regulatory expectations turning into greater stressful, should the market witness surprising shifts inside the way sterile fabric handling is designed and confirmed?
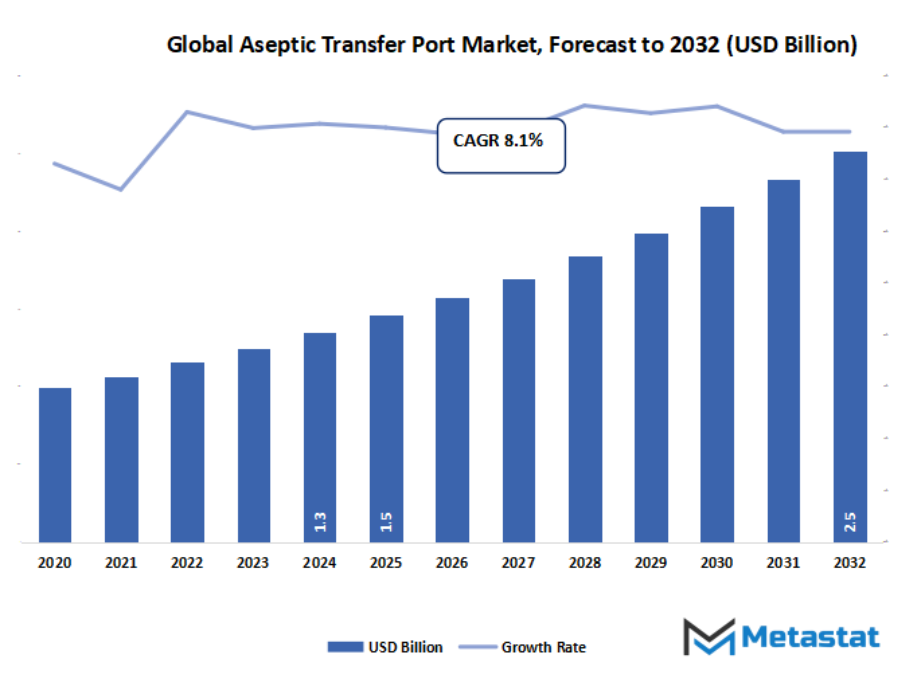
- The global aseptic transfer port market valued at approximately USD 1.5 Billion in 2025, growing at a CAGR of around 8.1% through 2032, with potential to exceed USD 2.5 Billion.
- Single-Use Aseptic Transfer Ports account for nearly 64.9% market revenues, driving innovation and expanding applications through intense research.
- Key trends driving growth: Strict regulatory requirements for sterility in pharmaceutical and biotech manufacturing., Growth in biopharmaceuticals and cell/gene therapies requiring aseptic processes.
- Opportunities include Adoption in emerging markets and CDMOs (Contract Development and Manufacturing Organizations).
- Key insight: The market is set to grow exponentially in value over the next decade, highlighting significant growth opportunities.
The global aseptic transfer port market will continue to shape a specialized industry that deals with the safe movement of materials where even the slightest exposure could disrupt an entire production line. Its future will stretch beyond conventional expectations, as manufacturers, laboratories, and advanced processing units will seek more than protection for their products; solutions that will support cleaner production cycles and reduce manual intervention. As equipment will grow smarter and more integrated, the market will be influenced by how companies rethink their internal processes, pushing them to adopt ports that work seamlessly with automated systems without breaking sterile boundaries.
Over the next several years, the industry will trend toward designs that adapt to new production layouts rather than forcing facilities to accommodate older structures. This trend will provide developers with the ability to experiment with lighter bodies, quicker locking mechanisms, and interfaces that will communicate directly with the monitoring systems. As global operations continue to grow, the second instance of the global aseptic transfer port market will be indicative of how companies will try to standardize practices across continents, requiring ports capable of withstanding various climatic conditions, regulatory pressure, and production speeds.
Geographic Dynamics
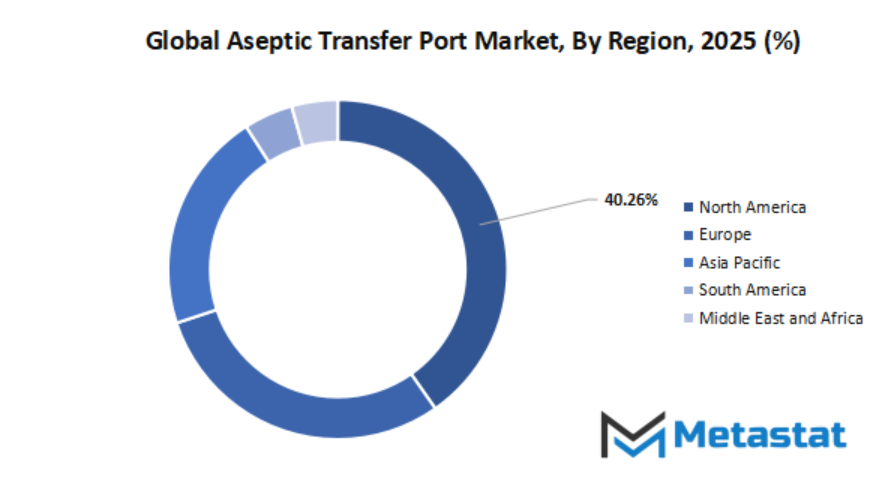
Based on geography, the global aseptic transfer port market is divided into North America, Europe, Asia-Pacific, South America, and Middle East & Africa. North America is further divided in the U.S., Canada, and Mexico, whereas Europe consists of the UK, Germany, France, Italy, and Rest of Europe. Asia-Pacific is segmented into India, China, Japan, South Korea, and Rest of Asia-Pacific. The South America region includes Brazil, Argentina, and the Rest of South America, while the Middle East & Africa is categorized into GCC Countries, Egypt, South Africa, and Rest of Middle East & Africa.
Market Segmentation Analysis
The global aseptic transfer port market is mainly classified based on Product Type, Application, End-User.
By Product Type is further segmented into:
- Single-Use Aseptic Transfer Ports
The demand for single-use aseptic transfer ports will continue to grow in the future within the global aseptic transfer port market, with production units shifting toward cleaner workflows and reduced turnaround time. As such, disposable structures will support safer handling of sensitive materials, support rapid batch changes, and lower contamination risks across high-precision production environments without compromising output standards.
- Multiple-Use Aseptic Transfer Ports
Reusable aseptic transfer ports will attract interest for operational stability in the long term. Robustness of the construction, readiness of repeated sterilization, and compatibility with advanced production lines will help such facilities that seek controlled material movement. Greater emphasis on systems of dependability will precipitate wider adoption, especially where stable manufacturing cycles require strong transfer mechanisms for regular operations.
By Application the market is divided into:
- Pharmaceutical Manufacturing
Advanced transfer solutions that support strict safety parameters will see continued adoption in pharmaceutical manufacturing facilities. Controlled transfer systems will strengthen material protection, reduce exposure concerns, and support continuous production runs. Such installations will guide the expansion strategies for manufacturers preparing for higher output requirements driven by rising therapeutic development pipelines.
- Biotechnology
Biotechnology environments will seek transfer solutions designed for sensitive biological materials. Controlled port systems will enable streamlined handling, reduce contamination challenges, and allow for smoother experimental progression. Greater biological innovation, ranging from genetic studies to the manufacture of advanced therapeutics, will foster broader acceptance of precise transfer mechanisms tailored to specialized workflows.
- Research Laboratories
Secure transfer structures that maintain material purity will support a wide range of experimental setups in research laboratories. Smoother transitions between stages will be possible because of flexible port configurations, which reduce procedural risk and let research teams focus on long-term innovation cycles that require dependable, contamination-free material movement within controlled laboratory workspaces.
- Others
Additional areas of application will implement aseptic transfer systems to support operations that require safeguarded material handling. The expanding category of production and specialized research requirements will spur facilities to integrate dependable options for transfers. Rising standards across broader industrial environments will also continue to shape adoption patterns for the operation of protected transfers.
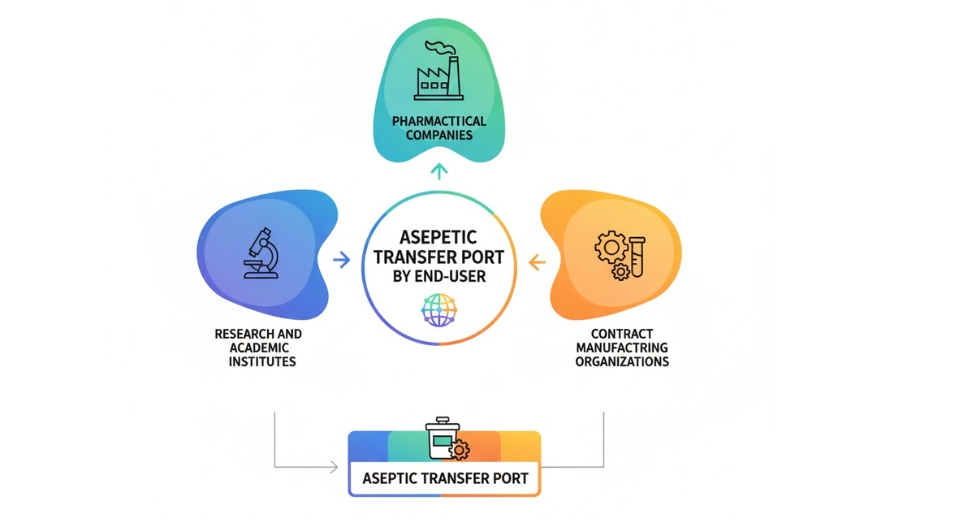
By End-User the market is further divided into:
- Pharmaceutical Companies
Aseptic transfer systems will be relied upon by pharmaceutical companies to ensure quality assurance across production lines. Having appropriate, secure handling structures in place will support reliable batch processing, lessen exposure concerns, and strengthen compliance strategies. Increasing medication development activities will continue to encourage investments in advanced transfer solutions that meet both regulatory expectations and expanding product portfolios.
- Research and Academic Institutes
Institutes of research and academics will seek out transfer systems that allow for the facilitation of controlled study conditions and the repeatability of experimental results. Precise solutions will help protect sensitive materials and facilitate workflow efficiency. Growing research diversity and project complexity will help define long-term preferences of stable transfer mechanisms.
- Contract Manufacturing Organizations
Advanced aseptic ports will be integrated into the facilities of contract manufacturing organizations to support scalable production programs. Material movement will be controlled to enable CMOs to meet the varied specifications of clients with reliable quality standards. Outsourced manufacturing demand will continue to drive increased utilization of transfer solutions designed for flexible, high-volume operations.
- Others
More end-user groups will look for aseptic transfer options with unique processing setups. Special industries requiring clean handling methods will look for adaptable systems that strengthen operational consistency. Attention towards contamination-free activities in the broader sectors will gradually expand adoption beyond the traditional pharmaceutical and research segments.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$1.5 Billion |
|
Market Size by 2032 |
$2.5 Billion |
|
Growth Rate from 2025 to 2032 |
8.1% |
|
Base Year |
2024 |
|
Regions Covered |
North America, Europe, Asia-Pacific, South America, Middle East & Africa |
Competitive Landscape & Strategic Insights
The global aseptic transfer port market will continue to forge beforehand as a space formed via each long-set up companies and more moderen contenders who are steadily gaining ground. It is this combination of lengthy-time worldwide gamers and up-and-coming nearby participants that has created a market where revel in meets clean perspective, permitting regular development as industries seek out safer and cleaner approaches to deal with sensitive materials. Companies dealing in pharmaceuticals, biotechnology, and advanced production will hold their dependence on aseptic transfer structures as it allows preserve product protection, helps regulatory necessities, and decreases the likelihood of infection in the course of production.
With years of technical enjoy and continuous innovation, well-recognized names encompass Getinge AB, Sartorius AG, Merck KGaA, Pall Corporation, and ChargePoint Technology Ltd. Their equipment has been widely commonplace because it supports the very best degrees of hygiene requirements and fits well into managed manufacturing strains. Complementing them, companies like Rommelag Kunststoff-Maschinen Vertriebsgesellschaft mbH, Aseptic Technologies SA, Central Research Laboratories (CRL), Flexicon Corporation, and Steris Corporation continue to add practical solutions that are helping manufacturers gain confidence in material handling. Each one of these has developed systems to make operators' jobs easier without compromising the quality of the product being transferred.
At the same time, newer and more region-focused players are infusing the market with fresh energy. Companies like BioPharma Dynamics Ltd, Bosch Packaging Technology, Meissner Filtration Products, Inc., Colder Products Company - CPC, Saint-Gobain Performance Plastics, AptarGroup, Inc., Ezi-Dock Systems Ltd, TASI Group, and Vanrx Pharmasystems Inc. are advancing designs that meet the changing needs of production environments. Their ideas often revolve around flexibility, ease of use, and improved integrations with state-of-the-art cleanroom settings. This mix of experience and new thinking is what will help the market stay responsive as industries increase their focus on safety and the purity of their products.
With growing attention being paid to sterile conditions and contamination control, aseptic transfer ports have become an indispensable element in advanced manufacturing. It is for this reason that industries, knowing even partial lapses in cleanliness can lead to huge losses, will always upgrade their systems. As standards continue to rise globally, more companies will seek dependable partners offering reliable equipment with practical support. In this way, both established leaders and emerging companies have an opportunity to get a stronger foothold by offering technology that helps clients be more productive while meeting strict safety expectations. Together, these international and regional players form a market that is going to continue pushing forward through new ideas, stronger safety needs, and rising global demand. With the likes of companies such as IMA Group adding to the progress of the global aseptic transfer port market, the industry will benefit from a steady mix of knowledge, competition, and innovation that fosters long-term growth.
Market Risks & Opportunities
Restraints & Challenges:
Implementation is complex and quite steeply-priced to validate:
In the future, the adoption pattern of the global aseptic transfer port market goes to be driven by using excessive financial commitments and needs for certain validation. Extensive trying out and qualification stages, coupled with strict compliance expectations, will gradual preliminary deployment, at least for facilities desiring long-term reliability. Increasing regulatory strain will similarly increase assignment timelines, and careful planning is required to ensure easy integration across advanced manufacturing setups.
Limited compatibility and standardization throughout one of a kind system providers:
A constrained amount of uniformity throughout the numerous systems will create technical friction within the global aseptic transfer port market. The dissimilarities in substances, size codecs, and engineering patterns will limit seamless product alignment. Cross-vendor collaboration will increase over time, however quick-time period uncertainty will persist, interfering with easy set up cycles and inspiring broader requires unified design expectancies in future production areas.
Opportunities:
Adoption in emerging markets and CDMOs:
Strong hobby from fast-growing areas and the expansion of CDMO networks will make stronger future possibilities for the global aseptic transfer port market. Increasing investment in sterile manufacturing, biologics manufacturing, and superior fill-finish preparations will retain to guide wider adoption. The growing call for for outsourcing will offer more room for novel transfer technology, ushering in long-time period transformation in worldwide manufacturing hubs.
Forecast & Future Outlook
- Short-Term (1–2 Years): Recovery from COVID-19 disruptions with renewed testing demand as healthcare providers emphasize metabolic risk monitoring.
- Mid-Term (3–5 Years): Greater automation and multiplex assay adoption improve throughput and cost efficiency, increasing clinical adoption.
- Long-Term (6–10 Years): Potential integration into routine metabolic screening programs globally, supported by replacement of conventional tests with advanced biomarker panels.
Market size is forecast to rise from USD 1.5 Billion in 2025 to over USD 2.5 Billion by 2032. Aseptic Transfer Port will maintain dominance but face growing competition from emerging formats.
What will make the difference in the market is how it will ignite deeper conversations about contamination control. The port will no longer be treated as a mere connecting component but a strategic enabler of higher output with less disturbance. By the time innovative facilities emerge with redesigned sterile zones, the third mention of the global aseptic transfer port market will point toward its transition from supportive technology to a defining feature of production environments of the future that will prioritize consistency, efficiency, and controlled material flow without compromising the stringency of sterile conditions.
Report Coverage
This research report categorizes the global aseptic transfer port market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global aseptic transfer port market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global aseptic transfer port market.
Aseptic Transfer Port Market Key Segments:
By Product Type
- Single-Use Aseptic Transfer Ports
- Multiple-Use Aseptic Transfer Ports
By Application
- Pharmaceutical Manufacturing
- Biotechnology
- Research Laboratories
- Others
By End-User
- Pharmaceutical Companies
- Research and Academic Institutes
- Contract Manufacturing Organizations
- Others
Key Global Aseptic Transfer Port Industry Players
- Getinge AB
- Sartorius AG
- Merck KGaA
- Pall Corporation
- ChargePoint Technology Ltd
- Rommelag Kunststoff-Maschinen Vertriebsgesellschaft mbH
- Aseptic Technologies SA
- Central Research Laboratories (CRL)
- Flexicon Corporation
- Steris Corporation
- BioPharma Dynamics Ltd
- Bosch Packaging Technology
- Meissner Filtration Products, Inc.
- Colder Products Company (CPC)
- Saint-Gobain Performance Plastics
- AptarGroup, Inc.
- Ezi-Dock Systems Ltd
- TASI Group
- Vanrx Pharmasystems Inc.
- IMA Group
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential




 US: +1 3023308252
US: +1 3023308252






