Oxygen Therapy Equipment Market To Reach $9,271.27 Million by 2032
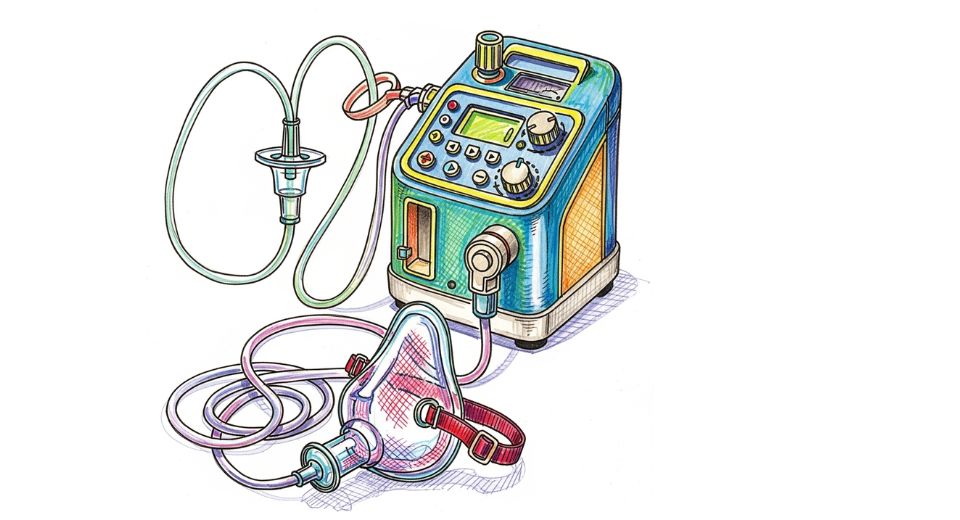
An in-depth story is revealed around the international Oxygen Therapy Equipment market report compiled by Metastat Insight that embarks on exploring dynamics which characterize present trends and predict likely movements of supply, deployment, and technological use. From an experienced filter of more than ten years, one can find a distinct picture emerging of trends in equipment usage, technological uptake, regulatory considerations, and supply chain adjustments all as part of the larger tapestry discussed in industrial and healthcare-briefed discourse relative to this market.
Distribution of oxygen therapy devices from varied settings acute-care hospitals and specialty clinics through to home-care configurations requires vigilant monitoring. Equipment types from concentrators to cylinders intersect with patterns of use that intersect by severity of respiratory diseases, availability of healthcare infrastructure, and character of care delivery systems. Providers delivering treatment in healthcare facilities depend upon systems that are strong in preparation for continuous use, whereas devices being used in personal or home settings prioritize portability, simplicity of use, and handling safety. The market evolves in intertwined strands: advancements in delivery technologies, innovations in efficiency and noise reduction, modification for backup power availability, interfacing with monitoring devices, and changes in manufacturing processes to adapt to materials shortages or logistical disruptions.
Strides to improve oxygen delivery systems go beyond technical details. Sustainability has become an issue, with dependency on materiality that minimizes environmental impact, energy efficiency in concentrators, and the integration of cleaner power sources energizing equipment in areas of poor grid stability. Supply chains evolve by moving production nearer to centers of demand or varying sourcing methods, such that interruptions like transport problems or raw material shortages do not translate into shortages of vital devices. Concurrently, service and maintenance models follow. Repair services, calibration practices, and training packages become essential elements of after-sales environments, to ensure ongoing reliability and safety in equipment operation.
Patterns of demand vary by geographic segment. Developing economies offer great potential for expansion, fueled by heightened awareness of respiratory health requirements, expanding healthcare facility upgrades, and growth in reimbursement systems. Conversely, established healthcare systems in developed areas urge improvement over expansion, which compels producers to seek device enhancements like reduced noise, lower footprints, and advanced usage monitoring. Public health programs targeting chronic respiratory ailments also drive demand shifts, as do seasonal respiratory stress episodes or emergency needs in cases of widespread respiratory disease outbreaks. Equipment makers counter with products that feature quick deployment, modularity for scalable application, and integrated monitoring capabilities to enable telehealth interventions.
Innovation strands flow through component miniaturization and electronic integration. Real-time adjustable oxygen concentration or flow rate feedback systems powered by sensors, battery technologies with greater portability, and smart connectivity allowing remote monitoring are ongoing areas of development. Collaborations between device manufacturers and software platforms enable remote patient management solutions that provide usage information, invoke alerts about maintenance needs, and enable monitoring of adherence. Such integrations through services and clinicians provide enhanced visibility into patient compliance, device operation, and timely intervention needs. Such technological advancements strengthen the respiratory care ecosystem without veering into clichéd nomenclature prevalent in traditional discourse.
Regulatory environments define the terrain through outlining performance standards, safety criteria, and market access approvals. Regulators across regions focus on quality assurance procedures, testing under diverse conditions, and alignment with global norms. Industry players adapt to changing expectations by streamlining production pipelines, strengthening documentation protocols, and cooperating with third-party verification organizations. Some regions impose further standards for home-use products, such as noise level restrictions, electromagnetic compatibility, and safe handling instructions. Such regulation guarantees stable device performance, enhances consumer trust, and facilitates more widespread adoption of oxygen therapy products.
Procurement trends indicate a trade-off between quality and cost-effectiveness. Healthcare professionals look for long-lasting units with low operating costs and good service terms. Family home care facilities value devices with ease of use that reduce the complexity of setup and provide reliable backup capabilities. Financing plans, leasing alternatives, and package service deals help purchasers control purchasing expenditures. Concurrently, maintenance networks expand to mitigate downtime hazards while ensuring safe operation.
Service ecosystems around equipment delivery consist of training modules for clinicians and caregivers, certification initiatives among technicians, and extension of remote diagnostic services. Virtual platforms for troubleshooting, augmented-reality repair guides, and tele-support services minimize the requirement for onsite intervention. With rising demand for after-sales service, service hubs and regional centers offer calibrated support, ensuring continuity and improving device longevity. This web of services makes supply-side approaches richer, nudging devices toward long-term useability and reliability.
Environmental demands, public health policy changes, and technological needs converge to set priorities for resource allocation. Governments balance strategic oxygen delivery unit stockpiling for emergency readiness, incentives to facilitate regional production, and regulation encouraging uptake at the community level of healthcare. Non-governmental organizations support by sponsoring deployment in underserved populations and orchestrating training initiatives. They drive demand curve patterns, particularly in regions with sparse infrastructure. Device developers, therefore, customize products to meet rugged conditions or applications featuring erratic utility supply, focusing on resilience and flexibility.
Competition becomes more global as companies compete to differentiate on the basis of innovation, localized care, collaborations with healthcare systems, and reputational resilience. Factors including brand equity, service network trust, and public health institution collaboration guide procurement choices. Companies that have a footprint across various markets benefit from an ability to go through regulatory layers, develop distribution infrastructure, and react effectively to changes in demand.
Through the vantage point of extensive industry exposure, the narrative of this market emerges not just as a catalog of technologies and distribution flows, but as a tapestry of strategic decision-making, adaptation, and collaborative progress. Evolving expectations from patients, clinicians, and regulators converge to shape solutions that meet immediate needs while anticipating future requirements.
Finally, the world oxygen therapy equipment market report presented by Metastat Insight culminates with validation of findings conceived through decades of experience and interaction with respiratory care technologies, interlacing supply dynamics, service ecosystems, technological integration, regulation harmonization, and emergent pattern demands.
Drop us an email at:
inquiry@metastatinsight.com
Call us on:
+1 214 613 5758
+91 73850 57479
 Agriculture
Agriculture
 Aerospace and Defense
Aerospace and Defense
 Automation & Process Control
Automation & Process Control
 Automotive and Transportation
Automotive and Transportation
 Banking & Finance
Banking & Finance
 Biotechnology
Biotechnology
1.png) Chemicals and Materials
Chemicals and Materials
 Consumer Goods
Consumer Goods
 Energy and Power
Energy and Power
 Food and Beverages
Food and Beverages
 Healthcare IT
Healthcare IT
 Information & Communications
Information & Communications
 Manufacturing and Construction
Manufacturing and Construction
 Packaging
Packaging
 Healthcare
Healthcare
 Electronics and Semiconductor
Electronics and Semiconductor
 Medical Devices
Medical Devices







 US: +1 3023308252
US: +1 3023308252






