MARKET OVERVIEW
In the cosmetic innovations, the US Clinical Cosmetic Testing Market stands as a crucial arena, playing a pivotal role in the development and validation of cosmetic products. This market is a dynamic space where scientific rigor intersects with the aesthetic aspirations of consumers. Its essence lies in the meticulous testing and validation processes that contribute to the safety and efficacy of cosmetic formulations before they grace the shelves.
One of the defining characteristics of the US Clinical Cosmetic Testing Market is its commitment to ensuring that cosmetic products meet the highest standards of safety and performance. This involves subjecting the formulations to a battery of tests, ranging from skin sensitization and irritation assessments to more complex studies evaluating product efficacy over time. The meticulousness of these evaluations underscores the industry's dedication to delivering cosmetic products that not only enhance beauty but also prioritize consumer well-being.
In the clinical cosmetic testing, human volunteers become indispensable partners in the journey of product development. These individuals, representative of the diverse demographics of the consumer population, participate in clinical trials that gauge the safety and effectiveness of cosmetic formulations. Their involvement is not merely a procedural step; it is a testament to the commitment of the industry to prioritize inclusivity and ensure that the products cater to the needs of a wide-ranging audience.
The US Clinical Cosmetic Testing Market also mirrors the advancements in technology, with cutting edge methodologies being employed to enhance the accuracy and efficiency of testing processes. From innovative imaging techniques that allow for precise measurements of skin parameters to state-of-the-art analytical tools that delve into the molecular intricacies of cosmetic formulations, technology acts as an ally in the pursuit of excellence within this market.
Furthermore, the market's significance extends beyond the laboratories and testing facilities. Regulatory bodies, such as the Food and Drug Administration (FDA), actively collaborate with industry stakeholders to establish and enforce standards that safeguard consumer health. The regulatory framework serves as a beacon, guiding the industry in its quest for continuous improvement and ensuring that the US Clinical Cosmetic Testing Market remains at the forefront of global best practices.
The US Clinical Cosmetic Testing Market is not just a sector; it is an indispensable force that ensures the harmonious convergence of science, innovation, and consumer well-being. Through rigorous testing, technological advancements, and a commitment to regulatory excellence, this market fortifies the foundations upon which the cosmetic industry builds its future. It is a testament to the dedication of industry players to not only meet but exceed the expectations of consumers, offering cosmetic products that are not just beautiful but are also backed by the rigor of clinical validation.
US Clinical Cosmetic Testing market is estimated to reach $732.9 Million by 2031; growing at a CAGR of 9.4% from 2024 to 2031.

GROWTH FACTORS
The growth of the US Clinical Cosmetic Testing market hinges on several pivotal factors that shape its trajectory. A fundamental driver is the escalating consumer demand for skincare and cosmetic products that exhibit tangible and measurable results. This burgeoning consumer inclination propels the imperative for stringent clinical testing, ensuring product claims are substantiated and align with the expectations of an increasingly discerning market.
Regulatory compliance, notably from entities such as the FDA, stands as another cornerstone in steering the market's upward trajectory. Heightened regulations necessitate cosmetic companies to undertake thorough clinical testing, a critical step in guaranteeing the safety and efficacy of their products. As governmental oversight becomes more stringent, companies find themselves compelled to invest in comprehensive testing processes to meet the evolving regulatory landscape.
However, amidst these growth factors, challenges emerge that could potentially impede the market's expansion. Clinical testing is a multifaceted process involving stages such as recruitment, ethical considerations, and data analysis. This complexity renders it a resource-intensive endeavor, particularly for smaller and emerging brands, potentially acting as a deterrent to market growth.
Ethical considerations, too, cast a shadow on the market's trajectory. Balancing the imperative for product testing with ethical concerns regarding human subjects becomes a delicate tightrope walk. This becomes especially pronounced when evaluating areas with potential sensitivity, such as eye and skin irritation. Striking a harmonious balance between the need for rigorous testing and ethical standards poses a significant challenge for market players.
The integration of emerging technologies, notably artificial intelligence and imaging, holds promise in revolutionizing clinical cosmetic testing. By leveraging these technologies, companies can enhance precision and efficiency, facilitating faster data collection and analysis while upholding the crucial standards of accuracy and safety. The incorporation of beauty tech into the clinical testing landscape represents a potential avenue for market expansion, offering lucrative prospects in the years to come. As technology continues to advance, the synergy between innovation and cosmetic testing may pave the way for a more efficient and effective market landscape.
MARKET SEGMENTATION
By Type
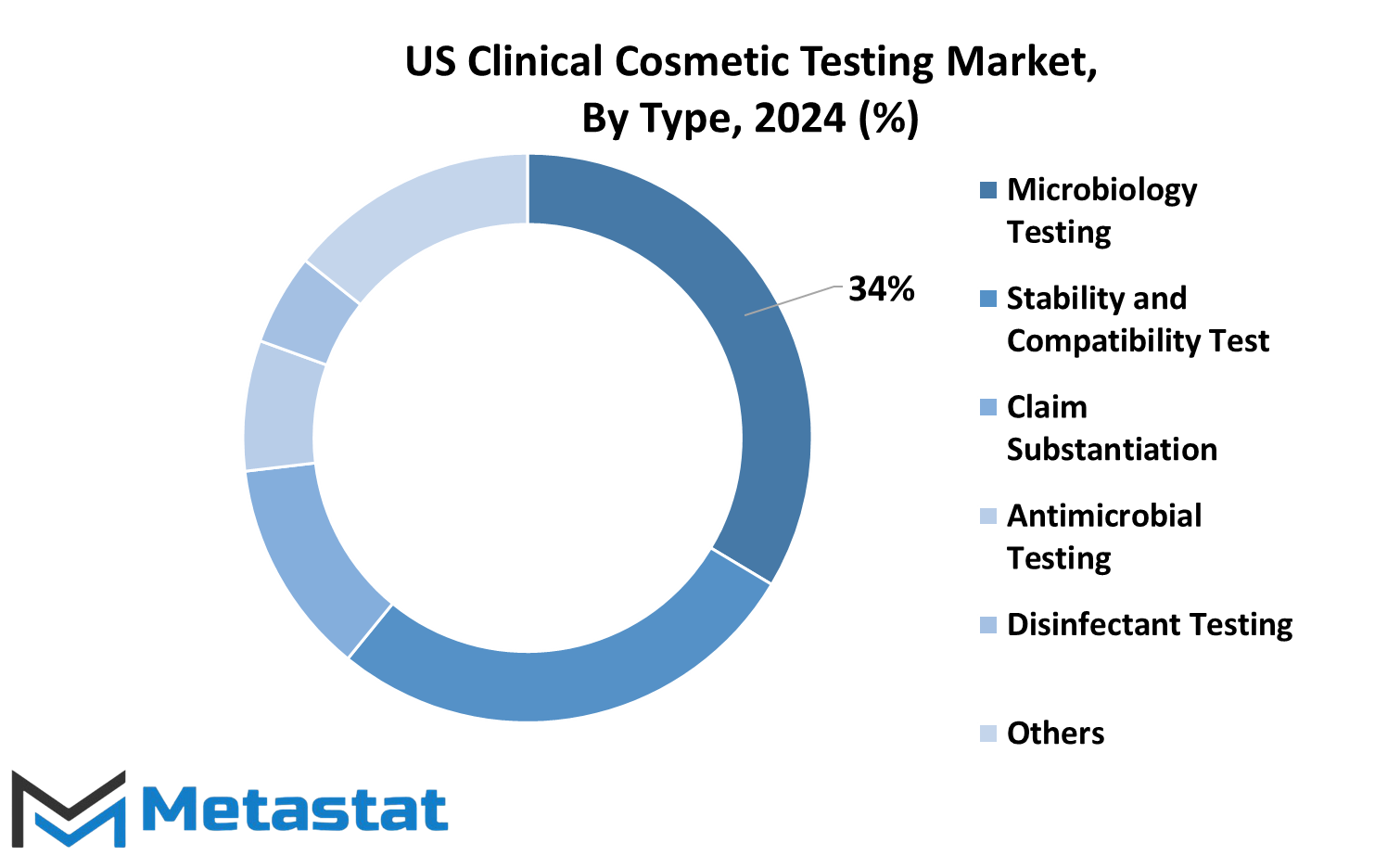
The US Clinical Cosmetic Testing market can be categorized into various types, each serving a distinct purpose in ensuring the safety and efficacy of cosmetic products. Among these types, Microbiology Testing, Stability and Compatibility Test, Claim Substantiation, Antimicrobial Testing, Disinfectant Testing, and Others play crucial roles in the assessment process.
Microbiology Testing forms a key component, focusing on the examination of cosmetic products for potential microbial contamination. This step is pivotal in safeguarding consumer health and preventing adverse reactions caused by the presence of harmful microorganisms.
Stability and Compatibility Tests are conducted to evaluate the product’s shelf life and its compatibility with different environmental conditions. This ensures that cosmetic items maintain their quality and effectiveness over time, meeting consumer expectations.
Claim Substantiation involves verifying the accuracy of the claims made by cosmetic products. This type of testing ensures that the promises regarding product benefits, such as anti-aging properties or skin hydration, are backed by scientific evidence, enhancing consumer trust.
Antimicrobial Testing is dedicated to assessing the effectiveness of preservatives in cosmetic formulations. Preservatives are essential to prevent the growth of bacteria and fungi in products, contributing to their overall safety.
Disinfectant Testing focuses on products intended for use in healthcare settings, ensuring their efficacy in eliminating harmful microorganisms. This type of testing is crucial, especially in the context of current global health concerns.
The category labeled as Others encompasses various additional tests that cater to specific needs within the cosmetic industry. These may include specialized assessments depending on the nature of the cosmetic product, providing a comprehensive approach to ensuring safety and quality.
The segmentation of the US Clinical Cosmetic Testing market by type highlights the multifaceted nature of the evaluation process. By addressing microbial concerns, stability issues, claim substantiation, antimicrobial efficacy, and disinfectant capabilities, the industry strives to uphold standards that prioritize consumer safety and satisfaction. The inclusion of diverse testing types underlines the comprehensive approach taken to assess the wide array of cosmetic products available in the market.
COMPETITIVE PLAYERS
The US Clinical Cosmetic Testing market is driven by key players such as Eurofins Scientific and Intertek Group plc. These companies play a crucial role in the industry, contributing significantly to the testing processes associated with clinical cosmetics.
Eurofins Scientific is a prominent player in the Clinical Cosmetic Testing sector. Their expertise lies in providing a wide range of testing services, ensuring that cosmetic products meet the required standards and regulations. Through rigorous testing protocols, Eurofins Scientific helps guarantee the safety and efficacy of cosmetic products before they reach consumers.
Similarly, Intertek Group plc is another major player in the Clinical Cosmetic Testing industry. Their focus on quality assurance and testing services has positioned them as a reliable partner for cosmetic companies seeking to validate their products. Intertek Group plc's contribution to the industry lies in its commitment to ensuring that cosmetic formulations adhere to regulatory guidelines and are safe for consumer use.
In the competitive landscape of Clinical Cosmetic Testing, both Eurofins Scientific and Intertek Group plc play pivotal roles in maintaining the integrity of cosmetic products. By leveraging their expertise, these key players contribute to the overall safety and quality of cosmetics available in the market.
The significance of these companies in the Clinical Cosmetic Testing market cannot be overstated. As consumers increasingly prioritize the safety and efficacy of cosmetic products, the role of key players like Eurofins Scientific and Intertek Group plc becomes even more crucial. Their commitment to stringent testing processes not only benefits the companies they serve but also ensures that consumers can trust the cosmetics they use.
The presence of key players, including Eurofins Scientific and Intertek Group plc, shapes the landscape of the US Clinical Cosmetic Testing market. Their contributions to quality assurance and adherence to regulatory standards underscore their importance in upholding the safety and efficacy of cosmetic products in the ever-growing cosmetics industry.
US Clinical Cosmetic Testing Market Key Segments:
By Type
- Microbiology Testing
- Stability and Compatibility Test
- Claim Substantiation
- Antimicrobial Testing
- Disinfectant Testing
- Others
Key US Clinical Cosmetic Testing Industry Players
- Eurofins Scientific
- Intertek Group plc
- SGS SA
- Charles River Laboratories
- Lucideon Limited
- WuXi AppTec
- Almac Group
- BluTest Laboratories, Ltd
- Nelson Laboratories, LLC
- Abbott Analytical Ltd.
- Pacific BioLabs, Inc.
- Accugen Laboratories, Inc
- Consumer Product Testing Company
- Cosmetic Testing Lab
- Creative Biolabs, Inc.
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential






 US: +1-(714)-364-8383
US: +1-(714)-364-8383






