MARKET OVERVIEW
The global cranial flap fixation market is a unique specialty within the broad medical device market, positioned near the need for precise, stable, and secure closure of cranial bone following neurosurgical procedures. Compared to other groups addressing general implants or orthopedic devices, this business is directly addressing cranial surgery only, in the form of trauma, tumor excision, and decompressive craniectomy. While technological advancements in surgical devices and imaging modalities have rendered a number of fields in neurosurgery more streamlined, fixation of cranial flaps remains a crucial aspect that will still determine the success or failure of surgery. Global Cranial Flap Fixation is not only about piecing bone together; it will be one area of concentration for technologies to converge on regenerative medicine, material science, and personalized patient treatment.
When this discipline continues to grow, its way will not be limited to traditional hardware appliances such as plates and screws anymore. Future breakthroughs will certainly challenge traditional clinical norms, introducing dynamic biomaterials with the capability of fitting in with biological settings and minimizing post-operative morbidity. The development of bioresorbable materials that disintegrate once the healing process is complete, or smart fixation systems capable of sensing intracranial pressure, will change the paradigm of cranial flap fixation. They will not only aim at safe closure of the skull but will make it possible to have frameworks that enable therapeutic, diagnostic, and protective capabilities in a single architecture.
The direction of the global cranial flap fixation market will also shift because patients and surgeons alike are placing increasing demands. There will be demand for low-profile devices and less invasive solutions, and manufacturers will be prompted to pursue ultra-light yet robust compounds that will not interfere with post-op scanning or neuro-monitoring. Research and development will also be aimed at developing personalized solutions from patient anatomy using 3D printing and digital modeling. These will place efficiency in operating rooms and reduce post-surgical complications that would otherwise lengthen hospital stays.
Extrapolating from the hospitals, the future of the market will be in the hands of external factors such as regulatory climates, reimbursement policy development, and ethics in smart implant utilization. As devices become ever more complex, regulation will be more stringent. However, this will lead to even greater standardization and safer procedure accessibility globally. Intellectual property disputes and international manufacturing will add additional levels of complexity, especially as emerging economies begin to have a more active role in specialist device production.
Long-term neurorehabilitation is one untapped area where the global cranial flap fixation market will break new ground. Fixation devices could move into being delivery systems for post-operative brain recovery, potentially communicating with neurostimulation devices or fluid drainage systems. The neural-hardware interface will continue to advance, opening up possibilities that extend far beyond the operating suite. With digital health on the rise, live data from brain implants could inform treatment streams, changing the way clinicians tackle recovery procedures.
In essence, the global cranial flap fixation market will move beyond its origin in cranial surgery. It will be a strategic platform where technology, medicine, and ethics intersect opening doors to possibilities beyond the ordinary and redefining the neurosurgical care of the future.
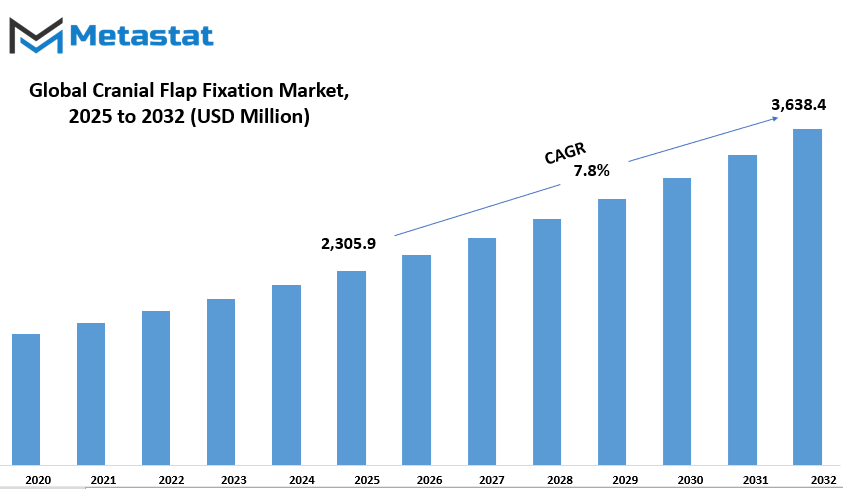
Global cranial flap fixation market is estimated to reach $3,638.4 Million by 2032; growing at a CAGR of 7.8% from 2025 to 2032.
GROWTH FACTORS
The global cranial flap fixation market is gradually but increasingly gaining traction due to a complementary combination of growing medical needs and emerging health developments. One major impetus of this market is the growing prevalence of traumatic brain injury and the heightened demand for neurosurgery.
Along with this, healthcare professionals are also showing a clear interest in newer and more reliable fixation technologies. The ease of utilizing materials not only safe but also effective in stabilizing cranial flaps in post-operative care is a main factor pushing the trend towards modern solutions. The devices fix the skull during post-operative care, helping patients recover without any chances of complications. Surgeons are increasingly demanding systems with easier application and less time in operation, which is also more convenient for hospitals in terms of efficiency.
However, aside from all these promising results, the market does actually possess a few formidable setbacks. The cost of cranial procedures and equipment remains costly, further making it difficult for most patients to receive the treatment they need. Especially where there is limited healthcare spending. Another issue is that there are not enough suitable facilities and skilled surgeons in most poorer or developing nations. These factors prevent the market from growing evenly across the globe.
There is a silver lining, however. Medical technology innovation is creating new possibilities. The most promising development in this sector is the advent of bioresorbable materials, which dissolve naturally in the body over time. They reduce the need for follow-up procedures and improve patient comfort. In addition, as developing countries continue to invest in developing their health care infrastructure, more and more hospitals can perform neurosurgical procedures, introducing new demand on cranial fixation devices. This trend is likely to recur because both public and private players put more focus on expanding access to quality care. Overall, despite some of the issues, the market is set to increase as awareness, innovation, and access keep improving.
MARKET SEGMENTATION
By Type
The global cranial flap fixation market is experiencing consistent growth, which is being fueled by rising surgery and development of neurosurgical instruments. Cranial flap fixation is an important aspect of neurosurgery utilized to fix the bone flaps in a craniotomy. It is required for protecting the brain and facilitating healing. With more and more individuals going for surgery to treat brain trauma, tumors, and other nervous system diseases, demand for effective and reliable fixation systems is gaining momentum.
The principal driver for the surge is the increasing number of trauma cases and neurological disorders globally. Most of them call for cranial surgeries, thereby making the demand for fixation devices increase.
The Cranial Plate category is now valued at $675.2 million and remains the most dominant category because of its reliability and popularity. Apart from plates, there are also other major categories such as Cranial Screws, Clamps, Loops, and Meshes. They all have specific functions to perform during surgery, depending on what the patient may need and what procedure is being undertaken. For instance, cranial clamps and screws are usually combined for additional support, and mesh devices are usually utilized in reconstructive surgery.
Technology is also contributing towards the growth of the market. Manufacturers are laying emphasis on creating products that are simpler to manage, take less time to operate, and provide improved outcomes. Some of these advancements being worked on include lightweight materials, improved design, and improved compatibility with imaging equipment. All these contribute not only in terms of efficiency of surgeons performing surgeries but also patient comfort and safety.
As health infrastructure develops in most countries and awareness of neurological treatment grows, more hospitals will increasingly look to embracing advanced cranial fixation procedures. This should create new opportunities for industry participants. On the whole, the global cranial flap fixation market will grow steadily since demand draws sustenance from a mix of medical necessity, technology, and wider access to advanced surgical practices.
By Material Type
The global cranial flap fixation market is growing slowly with the increasing number of neurological surgeries and traumatic brain injuries worldwide. Cranial flap fixation is a critical aspect of neurosurgery when a portion of the skull, i.e., the bone flap, is temporarily removed to access the brain. During surgery, the bone flap is locked into place through fixation systems to protect the brain and promote healing. The growing requirement for advanced medical device and improved patient outcomes is motivating hospitals and healthcare providers to spend money on stable fixation devices.
The market is divided based on material type as titanium alloy, ceramics, and polymers. Titanium alloy is one of the most frequently used materials for fixation of the cranial flap. It is extremely strong, yet light, and very biocompatible. It doesn't easily interact with body fluids or tissues and thus it's acceptable to be employed for a long duration of time. It can hold the bone flap in position effectively without irritating or rejecting it and thus it's a first choice for a surgeon. Ceramics are another fundamental material. Ceramics possess stability and strength. Ceramics are corrosion as well as degradation resistant, thereby ensuring long-term maintenance of fixation strength. While less frequently used than titanium, ceramics are gaining favor because they contain no metal, which is attractive to some patients, particularly those with metal allergies.
Polymers are also used for fixation of cranial flap. They are flexible and easily moulded, which proves useful in complex cases where the skull has unusual shapes. There are also polymers that can be absorbed, where they break down spontaneously in the body after some time. This has an advantage if a permanent implant is not needed. But while titanium would be too dense, polymers are not so strength-wise, so it would all depend on the needs of the surgery and how sick the patient is.
Each of these materials has advantages of its own, and selection often boils down to the needs of the patient and the skill level of the surgeon. As technology advances, we will undoubtedly see increasingly advanced materials being introduced for more optimal results. Attention continues to stay on patient safety, ease of use by surgeons, and durability of results, all which continue influencing the future of the cranial flap fixation market.
By Application
The global cranial flap fixation marketplace may be understood higher through looking at how it's miles used in extraordinary healthcare settings. Specifically, this market is split into most important software areas: hospitals and surgical centers. Each of these performs an critical position in how cranial flap fixation gadgets are used and how the marketplace grows.
Hospitals are one of the number one places where cranial flap fixation devices are needed. These clinical facilities manage a extensive variety of complicated surgeries, including those concerning the cranium and mind. Because of the variety and quantity of surgical procedures completed in hospitals, the call for for cranial flap fixation gadgets is frequently higher here. Hospitals have the infrastructure and assets to aid complex processes, which means that they depend heavily on dependable and powerful fixation methods. These devices help ensure that once a surgery where part of the cranium is eliminated or altered, it could be securely fixed returned in region. This is vital to defend the mind and allow for correct restoration.
On the alternative hand, surgical facilities are smaller centers that target unique types of surgical procedures, often with a extra outpatient recognition. These facilities are becoming more famous because of their performance and the capacity to offer specialized care. Even although surgical centers may not handle as many complicated instances as hospitals, they nevertheless use cranial flap fixation devices for procedures that require skull repair or reconstruction. Their developing presence inside the healthcare system method they may be an important part of the market. Surgical centers regularly aim to offer faster service with much less time spent in healing, so the fixation devices used have to be each powerful and well matched with short surgical tactics.
The global cranial flap fixation market depends on the needs of both these application areas. In hospitals they contribute significantly due to the wide range of performing surgery, while surgical centers offer special services and add to the market. Since more patients want a variety of surgical care, demand will continue to increase in both hospitals and surgical centers. This patient will affect the development and availability of new assessment equipment designed to improve results and surgical success. Understanding these application areas helps to see where the market is going and what kind of growth can be expected in the future.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$2,305.9 million |
|
Market Size by 2032 |
$3,638.4 Million |
|
Growth Rate from 2025 to 2032 |
7.8% |
|
Base Year |
2024 |
|
Regions Covered |
North America, Europe, Asia-Pacific, South America, Middle East & Africa |
REGIONAL ANALYSIS
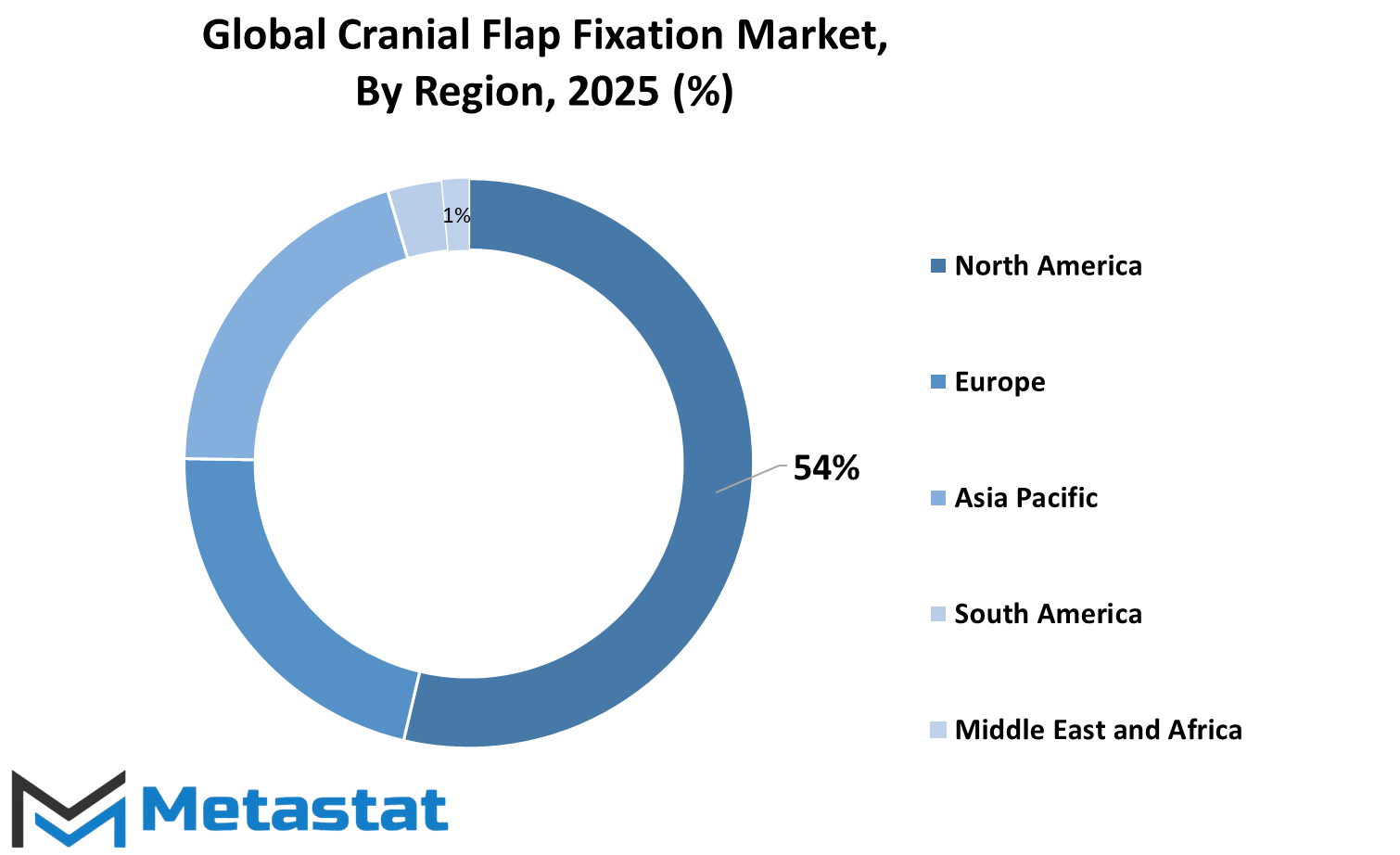
The global cranial flap fixation marketplace is split into numerous geographic regions to higher understand its distribution and increase capacity. These areas encompass North America, Europe, Asia-Pacific, South America, and the Middle East & Africa. Each region is in addition broken down into precise countries to offer more particular insights.
For instance, North America is separated into the US, Canada, and Mexico. Europe is split into the United Kingdom, Germany, France, Italy, and the relaxation of Europe. Similarly, the Asia-Pacific area covers India, China, Japan, South Korea, and different countries within the location. South America consists of Brazil, Argentina, and the relaxation of the continent, at the same time as the Middle East & Africa is made of the Gulf Cooperation Council (GCC) international locations, Egypt, South Africa, and different nations inside the region.
This geographic breakdown facilitates to focus on the one of a kind markets wherein cranial flap fixation strategies and products are used. North America, especially the U.S., is expected to remain a widespread marketplace due to its superior healthcare infrastructure and growing demand for neurosurgical approaches.
Europe also plays a key role, with nations like Germany and the UK contributing drastically due to their mounted clinical centers and research trends. In Asia-Pacific, the marketplace will in all likelihood see fast growth, driven by way of international locations including India and China wherein the healthcare area is expanding quick, and get admission to to advanced clinical remedies is improving.
South America, even though smaller in market length compared to others, will enjoy steady increase as healthcare systems improve and awareness about cranial fixation surgical procedures increases.
COMPETITIVE PLAYERS
The global cranial flap fixation market involves several important companies that play a major role in the industry. These key players work on developing and supplying the devices used for cranial flap fixation, which is a critical procedure in neurosurgery. Companies such as Medtronic, B. Braun, Johnson & Johnson, and Stryker are well-known in this field. They bring a wide range of products that help surgeons secure the bone flap after brain surgery, ensuring stability and proper healing.
Other significant companies in the market include Jeil Medical Corporation, Zimmer Biomet, NEOS Surgery, and Integra LifeSciences Corporation. These organizations contribute innovative solutions and advanced technologies that improve the safety and effectiveness of cranial flap fixation procedures. Their products are designed to meet the needs of patients and surgeons by providing reliable fixation methods that reduce complications during recovery.
Additionally, companies like OsteoMed, Kinamed Incorporated, Changzhou Huida Medical Instrument Co., Ltd., and evonos GmbH & Co. KG also hold important positions in the market. Each of these companies offers different types of fixation devices, such as plates, screws, and clamps, tailored to fit various surgical requirements. Their focus on quality and innovation allows them to compete successfully in this industry.
The presence of these key players highlights how competitive and diverse the cranial flap fixation market is. Their continuous efforts to improve product design and materials ensure that patients benefit from safer and more effective surgical treatments. The collaboration between these companies and medical professionals also helps to advance the overall standards of cranial surgery.
As the demand for better surgical outcomes grows, these companies will continue to invest in research and development. This will lead to new and improved fixation systems that simplify the surgical process and promote faster healing. The ongoing competition among these players will push the industry forward, benefiting both surgeons and patients worldwide.
In summary, the global cranial flap fixation market is shaped by several leading companies that focus on delivering high-quality fixation devices. Their commitment to innovation and quality helps improve surgical results and patient recovery. The industry will keep evolving as these key players work towards making cranial flap fixation safer and more efficient for everyone involved.
Cranial Flap Fixation Market Key Segments:
By Type
- Cranial Plate
- Cranial Screw
- Cranial Clamp
- Cranial LOOP
- Cranial Mesh
By Material Type
- Titanium Alloy
- Ceramics
- Polymers
By Application
- Hospitals
- Surgical Centers
Key Global Cranial Flap Fixation Industry Players
- Medtronic
- B. Braun
- Johnson & Johnson
- Stryker
- Jeil Medical Corporation
- Zimmer Biomet
- NEOS Surgery
- Integra LifeSciences Corporation
- OsteoMed
- Kinamed Incorporated
- Changzhou Huida Medical Instrument Co., Ltd.
- evonos GmbH & Co. KG
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential





 US: +1 3023308252
US: +1 3023308252






