MARKET OVERVIEW
The Global Computer System Validation market is shaping the technological landscape for industries governed by strict regulatory compliance and operational efficiency. As companies adopt complex digital solutions into their own workflows, the onus is on assurance that these systems are functioning correctly and securely. Pharmaceuticals and healthcare, manufacturing, and financial services industries all practice stringent validation in order to maintain data integrity, endure system reliability, and meet regulatory prescriptions. This market, thereby, provides risk cushion opportunities in mitigating the risks that are attached with digital transformations whereby software applications, cloud-based platforms, and automated systems are verified against specified functional and performance criteria.
As industries become ever-more capitalized in the fields of automation, the Global Computer System Validation market would be considerably broadening its scope to integrate and include new technologies. The traditional validation practices mainly catered to legacy systems and on-premise infrastructures; companies are now slowly adapting to cloud-based environments and AI-driven applications. These require validation techniques advanced enough to assess security, correctness, and performance of interconnected systems. The demand for validation services will keep increasing as industries continue embracing artificial intelligence, blockchain, and machine learning technology. Regulatory emphasis on data transparency, cybersecurity, and risk management necessitates the validation of these technologies.
Perhaps the most defining element of the Global Computer System Validation market is its conformity to highly stringent compliance frameworks. Regulatory authorities such as the FDA, EMA, and other global bodies enforce strict guidelines to see to it that businesses maintain data integrity and operational reliability, which the Global Computer System Validation market will be traversing. Organizations in this space will be tracking evolving regulations and learning about validation procedures that allow for frequent updating of compliance standards. With growing complexity in regulatory regimes, these organizations will be looking for automated validation tools to streamline compliance processes whilst unfastening operational bottlenecks.
The increasing shift toward cloud computing is yet another dimension that will change the Global Computer System Validation market. Cloud-based validation solutions promise to provide more flexibility in terms of scalability, while ensuring compliance enabled remote validation of systems. However, the same cannot be said for classical validation approaches, whose application will cease with cloud adoption for SaaS and IaaS by corporations. This transformation will in turn encourage enterprises to maximize investment in automated validation systems that would integrate with the cloud with minimum human intervention and maximum observational coverage.
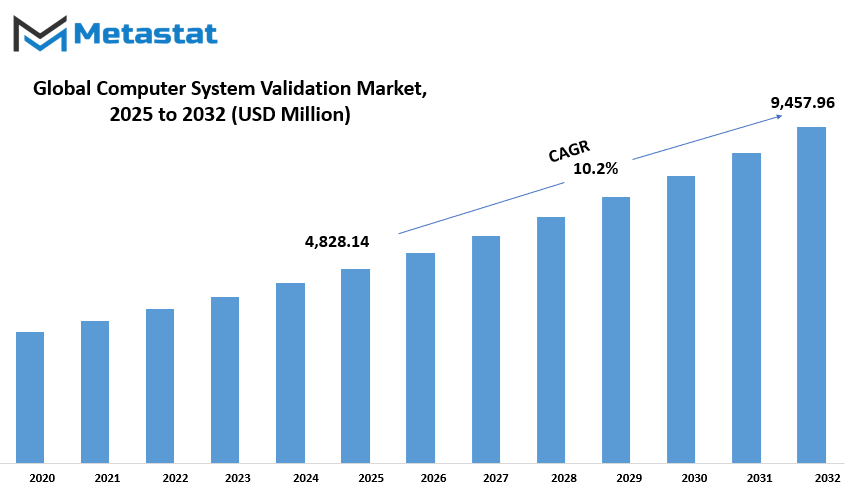
Global Computer System Validation market is estimated to reach $9,457.96 Million by 2032; growing at a CAGR of 10.2% from 2025 to 2032.
GROWTH FACTORS
With organizations now concentrating more on compliance, efficiency, and accuracy in their affairs, the Global Computer System Validation market is developing. Validations are emphasized by the regulatory authorities of the pharmaceutical and healthcare sectors so that computerized systems dealing with sensitive information maintain their integrity. Regulatory compliance is fast growing into an important motivation for the growing number of computer system validation requirements. Companies must prove that their software and digital systems work according to the records, thereby reducing errors that may affect the safety of any patient, the confidentiality of any data, or the efficiency of any operation. Therefore, validation solutions are being heavily invested in to ensure industry and regulatory requirements are being met.
Secondly, one of the significant factors for increasing market growth is the growing acceptance of automation in validation solutions. Traditional validation processes are time-consuming and, therefore, sometimes lead to errors due to human factors. This is where automation comes into play by streamlining these processes, thereby enhancing accuracy and efficiency and creating less burden for manual labor. Companies incorporate AI and ML for better optimization and consistency in the validation framework. Documentation, testing, and compliance tracking can be automated, enhancing productivity and operational integrity. This shift toward the use of automation is bound to spur further developments in the field.
Despite the advantages, there are some limitations for the market. Finance is one of the primary challenges faced in the implementation of computer system validation. Especially in those cases of small and medium enterprises, for them the initial capital investment along with cost of operation and maintenance may be too much of a burden. Furthermore, complexities associated with validation procedures, in which implementing the solutions can be hampered, give rise to one more challenge in computer system validation. Specialized skills are needed to navigate through the regulatory requirements as well as technical requirement processes of validation system, which could confine the adaptability. Besides, with the exception of maybe a few regions, within emerging economies, there is still very low awareness regarding the importance of validation among organizations, which limits the growth of the market.
However, a lot of business opportunities lie. Most of the prominent opportunities for growth lie in the rapid acceptance of cloud-based validation solutions. Cloud technology enables the organization to remotely manage their validation processes with minimal investment in infrastructure while improving accessibility. Another major trend being witnessed in the industry is AI automation, as companies are keen to find quicker means to meet their compliance requirements without too much manual intervention. The need for stronger validation frameworks is now being realized by different industries outside healthcare such as IT and manufacturing.
MARKET SEGMENTATION
By Type of Service
Global Computer System Validation is an important application in validating that computer systems are functionally reliable, secure, and uphold various regulatory requirements in different industries. The service requirement for validation has increased with the ever-growing dependency on digital processes in core business operations, data management, and customer interactions. Companies should ensure that their systems are error-free, efficient, and well-configured to meet industry standards so that they do not face compliance issues, security risks, and operational failures. Validation ensures that organizations can maintain data integrity, while their computer software and hardware corollate with defined requirements under various operating conditions.
Service type forms a big component of this market. Consulting services account for a significant portion, some US$1,516.93 million, since organizations seek expert guidance in regulatory compliance and system implementation. Testing services make up another important segment, as they help companies prove that their computer systems behave in the intended manner with the least chance of interruption of business should they fail. Documentation services ensure that all required records, protocols, and reports are kept in line for compliance, affording the organization with a systematic approach to validation of their system. Correspondingly, training services equip the employees with the skills to carry out best practices and understand the validation process. Other specialized services would cater to the organizations on specific validation needs uniquely arising from sector-based requirements.
The adoption of cloud computing, artificial intelligence, and automation has magnified the need for proper validation. Integrating technology into the workflow processes amid the business models is not easy; businesses need to make sure the different systems cooperate and work according to regulations. The very stringent validations in healthcare, pharmaceuticals, and finance sectors, with respect to their handling of data, are mandated by regulations governing their activities. These industries may face dire consequences due to any mishap or breach in security; therefore, system validation would set its precedence.
Even so, there is still a huge struggle due to implementation costs and challenges for businesses in complying with validation for fast-changing technological trends. Notwithstanding, with regulatory bodies tightening compliance standards and industries realizing the long-term benefits accruing from validated systems, the market is poised for expansion. And perhaps as a bonus, systems are likely to perform better, be more secure, and generate efficient operations-uninterrupted and error-free-thanks to validation boosters.
By Application
The Global Computer System Validation market is fundamental to complying with regulations in industries where accuracy and data integrity are the hallmarks of doing business. This market regulates that computer systems used in various applications are working according to their intended purpose and are thus reducing risks associated with data security and operational failure and violation of the policies of compliance. Industries such as pharmaceuticals, biotechnology, medical devices, and clinical research are key players where these validation processes serve as very important cogs in ensuring quality assurance and regulatory adherence. Validation of computer systems stands out as basic regulatory compliance. It is therefore a prerequisite for improving operational efficiency and reliability.
Pharmaceutical companies validate systems for drug development, manufacturing, and distribution. They track data, assure product consistency, and uphold stringent regulatory requirements. If validation were to fail, however, huge amounts of money could be lost, products could be recalled, or a legal case placed against the company. In biotechnology, system validation is vital for key processes such as maintaining accurate experimental records, reproducibility, and protecting intellectual property. A breakdown in proper validation within this industry could impact the integrity of the research and cause delays in product approvals.
Medical device regulations require validated systems to ensure the marketing of safe and effective devices. WIth these systems, safety and effectiveness studies can be designed, executed, and submitted for regulatory purposes to show that devices function as intended in numerous environmental conditions. In order to protect patient data, maintain protocol adherence, and assure ethical standards, clinical research organizations running clinical trials and analyzing the results are using validated software. The integrity of trial data is at the core of regulatory approval and ultimately the success of any new medical interventions.
Aside from these sectors, computer system validation is very helpful to several other sectors that hold massive amounts of sensitive data. All of these sectors have strict guidelines under the authorities that oversee them worldwide-fda and ema-for validation. Growing changes in technology have made it possible for companies to come up with automated solutions to speed up validation processes so as to minimize user error and increase compliance efficiency. This will give newborns, after training firms on advanced validation methods for their systems, the ability to remain in a state of continued compliant operation while adapting to an ever-rising level of regulatory demands, thus offering great competitive weightage.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$4,828.14 million |
|
Market Size by 2032 |
$9,457.96 Million |
|
Growth Rate from 2025 to 2032 |
10.2% |
|
Base Year |
2025 |
|
Regions Covered |
North America, Europe, Asia-Pacific, South America, Middle East & Africa |
REGIONAL ANALYSIS
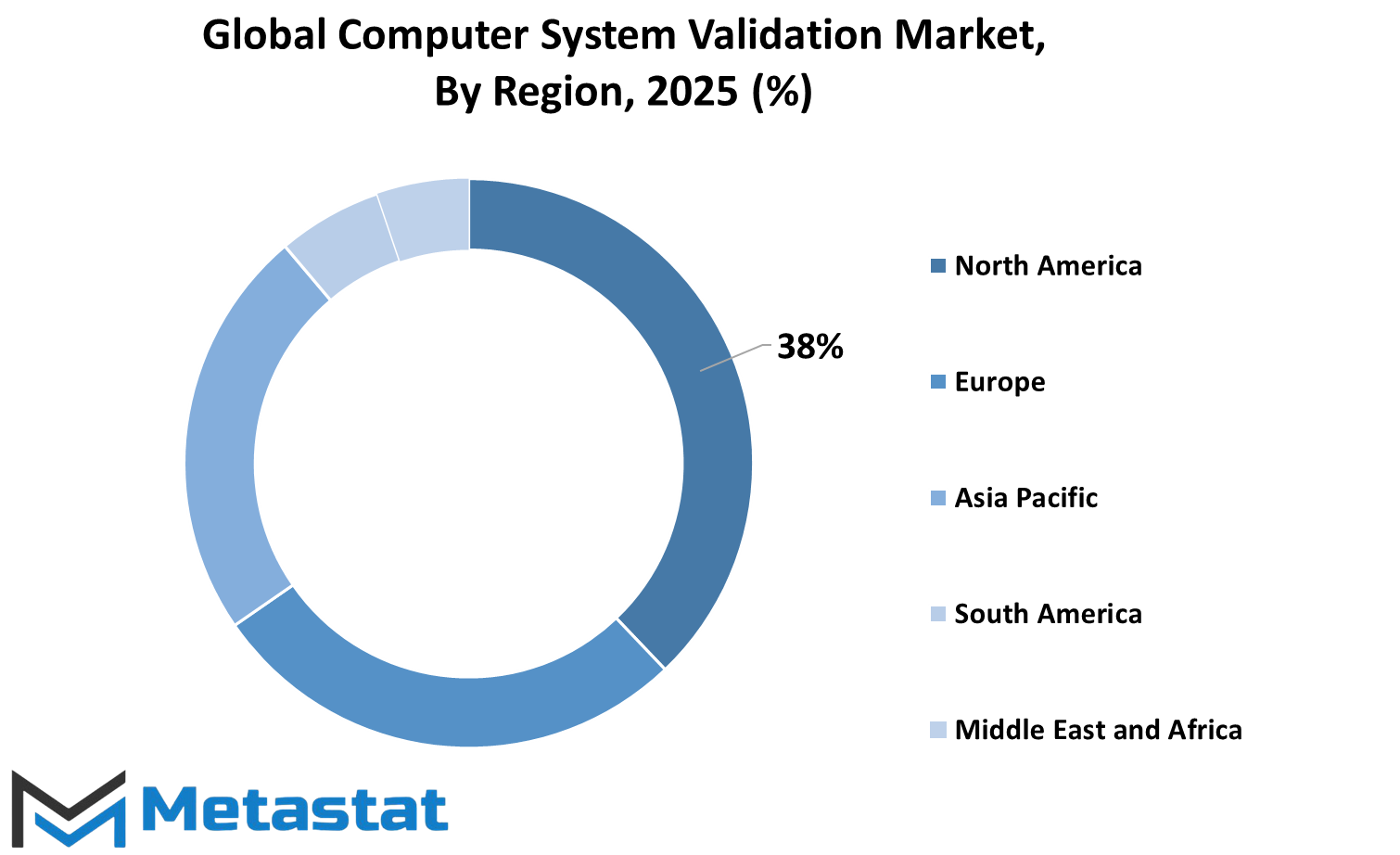
The Global Computer System Validation Market can be segmented geographically into the following regions: North America, Europe, Asia-Pacific, South America, and the Middle East and Africa. The North American market segment is further divided into the U.S. market, Canadian market, and Mexican market. Europe consists of the United Kingdom, Germany, France, Italy, and the rest of the countries in Europe. In Asia and the Pacific, the major markets include India, China, Japan, South Korea, and other countries in the Asia-Pacific region. In South America, arguably Brazil and Argentina are the more important markets, with relevance to some extent for the rest of South America. In the United Kingdom, all countries of the Gulf Cooperation Council (GCC), Egypt, and South Africa are included.
Each geographic segment has its specialization and specificity when it comes to the trends and advances of computer system validation. North America, being a strong regulatory and technology innovation region, remains the key player that trends adoption, followed closely by Europe with its stringent compliance requirements affecting the market trend. Come Asia Pacific, it appears to be a developing and promising region for growth owing to the growing attention on the digital transformation of countries like India and China. South America and the Middle East and Africa are increasingly growing but at a different pace in terms of industries seeking to establish their systems whereby they will be contributing.
Now, this division helps in learning how regional influences tailor strategies for companies. Therefore, as industries across the globe continue to develop their digital infrastructures, the demand for reliable validation solutions will forever be one of the critical determining factors on compliance and efficiency.
COMPETITIVE PLAYERS
Market for Global Computer System Validation is important for reliability, efficiency, and compliance of computerized system in the pharmaceutical, biotechnology, and healthcare industries. Increasing regulatory requirements require companies to validate their systems to industry standards to avert risks and protect data integrity. Validation ensures that software and hardware systems are functioning, avoiding those that might interfere with safety, productivity, and application of laws, such as FDA 21 CFR Part 11 and EU Annex 11.
Validation assesses the software lifecycle, from its birth to taking operation, determining if the curve is indeed in accordance with the imposed set of quality standards. The companies apply scientific methodologies, such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), for the systematic scrutiny of their systems. Hence, system validation will ensure compliance, smoothen operation, and eliminate vulnerabilities before they can disrupt business conduct. In the absence of a proper validation mechanism, organizations will entice regulatory scrutiny and operational interruptions, with financial penalties being the last straw, which makes it an investment worth considering for sustainable growth.
With the penetration of digital technologies into highly secured industries and sectors that require high precision, computer system validation has developed high demand. Automation, artificial intelligence, and cloud-based solutions are changing the validation scenario toward being cost-effective and fast. Now companies are starting to integrate validation with system development life cycles rather than treating it as a standalone compliance exercise. This will allow for continuous monitoring and improvement, cutting downtime and ensuring system reliability.
Several major players drive growth and innovation in this market. Siemens AG, Kevin Technologies, IncepBio, and Honeywell International Inc. offer advanced validation solutions customized for different industry needs. Microsoft Corporation and Oracle Corporation also provide software platforms that enable organizations to effectively manage validation processes. Ofni Systems and ProPharma are experts in compliance-driven validation services that will ensure that companies meet any stringent regulatory requirements. Additional leading providers include Biotech, PharmOut Pty Ltd, Xybion Digital Inc., Deloitte, Thermo Fisher Scientific Inc., and Cognizant Technology Solutions, all of which offer complementary expertise in system validation, risk management, and regulatory compliance.
Computer System Validation Market Key Segments:
By Type of Service
- Consulting Service
- Testing Service
- Documentation Service
- Training Service
- Others
By Application
- Pharmaceuticals
- Biotechnology
- Medical Devices
- Clinical Research Organization
- Others
Key Global Computer System Validation Industry Players
- Siemens AG
- Kevin Technologies
- IncepBio
- Honeywell International Inc.
- Microsoft Corporation
- Ofni Systems
- ProPharma
- Oracle Corporation
- Biotech
- PharmOut Pty Ltd
- Xybion Digital Inc.
- Deloitte
- Thermo Fisher Scientific Inc.
- Cognizant Technology Solutions
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential






 US: +1-(714)-364-8383
US: +1-(714)-364-8383






