Global Chemiluminescence Immunoassay Market - Comprehensive Data-Driven Market Analysis & Strategic Outlook
The global chemiluminescence immunoassay market along with its industry, tell a story that has been going on for a long time before automated diagnostic systems crowded modern laboratories. At the end of the 1900s, researchers wanted new non-radioactive assays and more sensitive methods to diagnose diseases in hospitals and laboratories. One of the first steps in luminescent enzyme reactions was a whole new detection method: light instead of radioactivity could be used to indicate the presence of a disease. This transition laid the groundwork for the eventual development of what we now call the market, which supports the whole spectrum of clinical labs, pharma developers, and public health programs across the globe.
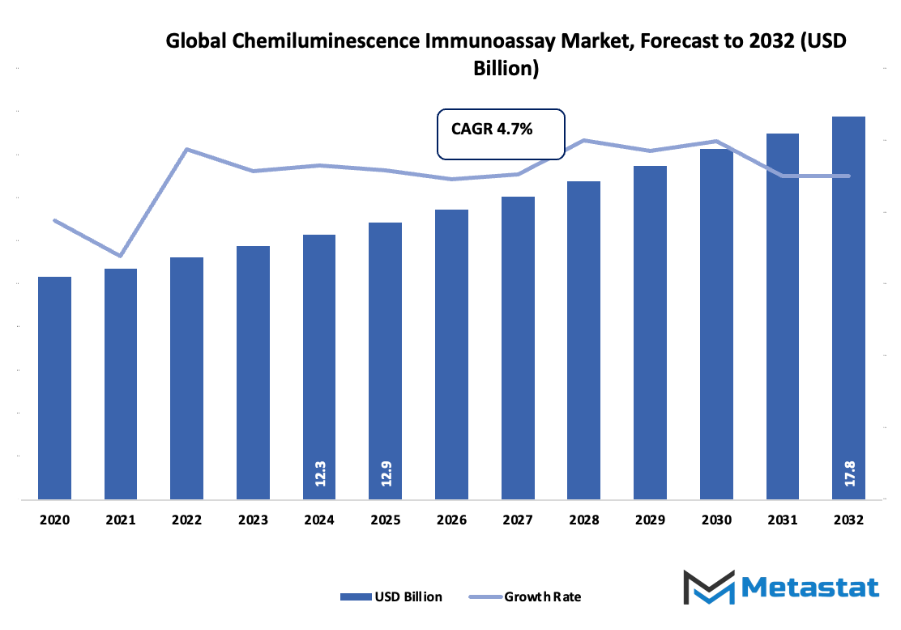
- The global chemiluminescence immunoassay market was valued at nearly USD 12.9 billion in 2025, showing a CAGR of approximately 4.7% until 2032 and a possibility to surpass USD 17.8 billion.
- Instruments are responsible for almost 43.5% of the market share, and as a result, through intense research, innovations are being made and applications are being expanded.
- Some of the key trends that are driving the growth are: increasing demand for high-sensitivity and quick testing, rising chronic and infectious diseases that need accurate detection.
- The opportunities are: connecting with automated and point-of-care testing systems to make diagnostics quicker and more decentralized.
- The key insight here: the market is going to become very valuable very fast in the next ten years, thus, there are considerable growth prospects.
In the beginning, the technology was still in the experimental stage and was only allowed in advanced research facilities. The machines were costly and hard to maintain. The main change happened when instrument manufacturers launched small analyzers with the capacity to perform several tests simultaneously. Test automation made the whole process quicker and more uniform, so hospitals were already investing in these systems to enhance medical diagnostics. Another big step forward was taken in the early 2000s when companies started to roll out user-friendly cartridges alongside pre-calibrated reagents. This allowed even the smaller labs, such as regional clinics, to use the advanced chemiluminescent detection technology without the need for specialized staff.
The evolution of healthcare standards led to a change in testing toward those that would identify diseases at the very earliest stage. This, in turn, was a persistent call for designers to come up with systems that had extremely precise accuracy and very quick turnaround time. The large scale of early detection of chronic and infectious conditions prompted the researchers to stretch the test menus right from hormones and cardiac markers to oncology and autoimmune screening. Digital connectivity, which comprised cloud-based data systems, also got into the picture and changed the whole paradigm of how laboratories managed the patient information. Instruments began to communicate directly with electronic medical records so that clinicians could take quicker decisions and at the same time reduce the amount of paperwork.
The regulatory authorities have also been instrumental in defining and setting the standards of the day. The requirements for traceability, quality control, and patient safety were tough but pushed the manufacturers to perfect their instruments and reagent kits down to the very last detail. These transitions were instrumental in the development of trust between laboratories and clinicians. The future of the global chemiluminescence immunoassay market appears to be one of a continuing shift toward faster workflows, less sample usage, and closer ties with data analytics.
Market Segments
The global chemiluminescence immunoassay market is mainly classified based on Product Type, Technology, Sample Type, Application.
By Product Type is further segmented into:
- Instruments: Instruments utilized for chemiluminescence immunoassay yield measurement precision and facilitate laboratory operations that are supportive of diagnostics. Modern analyzers make testing quicker and more effective. Manual handling is less likely to cause mistakes and it also leads to similar results. Design improvements are constant which allows for a wider range of tests to be offered and a changeable sample capacity to be used, thus making it possible for laboratories to handle increased testing volumes with reliable performance.
- Consumables: Consumables are such things as reagents, cartridges, and other laboratory materials that are necessary for the execution of every chemiluminescence immunoassay procedure. Dependable consumables make sure that signal detection is consistent and that outcome generation is precise. The regularity of the supply of consumables supports testing without stops and also cuts down on the time needed for diagnosis when testing is done in facilities. The demand for quality-controlled consumables increases along with the rising frequency of testing in various clinical and research environments.
By Technology the market is divided into:
- Chemiluminescence Enzyme Technology (CLEIA): Chemiluminescence Enzyme Technology uses enzyme reactions to produce light signals, which allows for the highly sensitive detection of targets such as hormones or disease markers. The high sensitivity of such tests enables laboratories to trust the detection of even the smallest concentrations. The faster processing time reduces the waiting period for clinical interpretation, thus supporting the establishment of better patient care decisions in the healthcare systems.
- Electrochemiluminescence Immunoassay (ECLI): Electrochemiluminescence Immunoassay combines the light emission with the electrical stimulation to boost the sensitivity and simultaneously to reduce the background interference. The noise reduction makes for more reliable measurements. The laboratories welcome the possibility to analyze difficult test targets with a high degree of accuracy. The stable signal generation backs up the quality control during the routine and advanced diagnostic investigations in the various clinical environments.
- Microparticle Chemiluminescence Immunoassay: Microparticle Chemiluminescence Immunoassay is based on the use of very small particles that promote surface interaction during the tests. The larger surface area for binding enables the laboratories to capture a larger number of target molecules, which in turn leads to greater light production. The quicker binding process leads to a shorter testing time, whereas the increase in signal intensity results in more accurate interpretation of biologically relevant biomarkers.
By Sample Type the market is further divided into:
- Blood: Blood samples remain a primary choice for chemiluminescence immunoassay because many biomarkers circulate within blood. A blood sample supports broad diagnostic coverage ranging from infectious screening to hormone monitoring. Blood-based testing provides strong reliability and wide clinical acceptance within hospitals, laboratories, and research centers.
- Urine: Urine samples support non-invasive collection, creating comfort for individuals who may not tolerate blood collection. Urine assists in detecting specific metabolic or hormonal indicators. Laboratories apply urine testing in diverse conditions where biomarkers naturally pass through the urinary system, supporting early detection and routine monitoring.
- Saliva: Saliva collection delivers a painless and convenient option for frequent monitoring. Saliva naturally contains multiple biomarkers that reflect metabolic or hormonal activity. Saliva sampling supports community-based screenings and at-home testing, opening access to diagnostics without specialized staff or facilities, while still maintaining dependable result quality.
- Other Sample Types: Other samples include tissue extracts or cerebrospinal fluid used when targeted biomarkers exist outside blood, urine, or saliva. Each sample type supports specialized diagnostic needs and helps clinicians investigate complex medical conditions. Expanded sample options increase flexibility and strengthen decision-making during challenging or rare disease assessments.
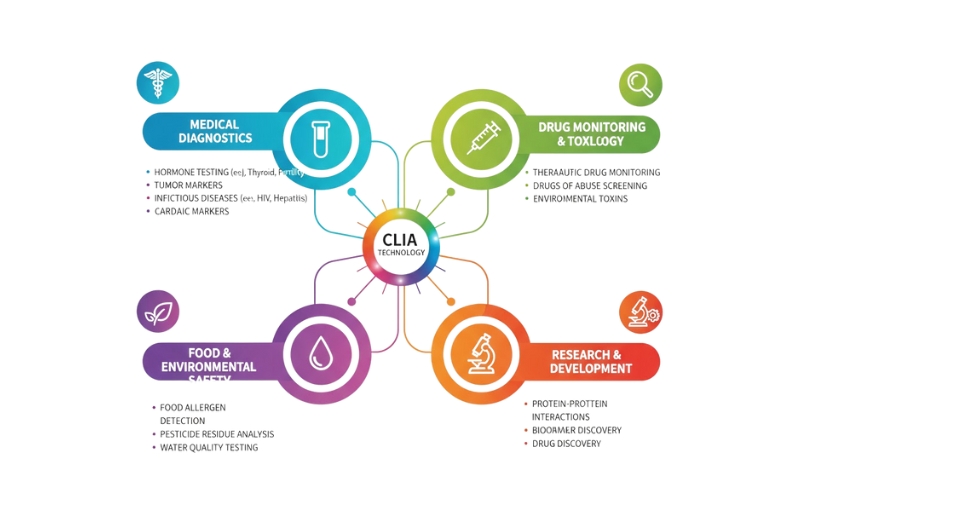
By Application the global chemiluminescence immunoassay market is divided as:
- Infectious Disease: Chemiluminescence immunoassay supports infectious disease testing by detecting antigens or antibodies linked to pathogens. Rapid identification assists healthcare teams in acting early during treatment decisions. High sensitivity supports screening programs and public health monitoring. Widespread usage helps minimize disease transmission through timely detection and actionable diagnostic insight within communities.
- Endocrinology: Endocrinology testing monitors hormones that regulate body functions. Chemiluminescence immunoassay assists in detecting hormonal imbalance associated with thyroid disorders, fertility concerns, and metabolic challenges. Accurate hormone measurement guides treatment planning and supports ongoing monitoring. Reliable hormone evaluation strengthens clinical guidance during both routine assessments and long-term care management.
- Oncology: Oncology applications focus on detecting tumor markers linked to cancer progression or treatment monitoring. Early marker detection helps medical teams understand disease stage and plan therapy. Chemiluminescence immunoassay enables frequent monitoring, helping to evaluate treatment response and track potential recurrence with strong sensitivity.
- Cardiology: Cardiology diagnosis benefits from chemiluminescence immunoassay because cardiac biomarkers indicate heart strain or tissue damage. Rapid confirmation of marker levels assists emergency teams during suspected heart events. Consistent measurement helps track recovery progress and supports long-term cardiovascular management, improving decision pathways during critical care.
- Allergy Diagnostics: Allergy diagnostics rely on detecting specific immune responses to allergens. Chemiluminescence immunoassay helps laboratories quantify allergen-specific antibodies with accuracy. Precise measurement supports the identification of triggers and guides personalized treatment strategies. Strong sensitivity aids clinicians in differentiating between similar reactions and designing tailored plans.
- Other Applications: Further applications include monitoring drug levels, autoimmune disorders, and research studies. Chemiluminescence immunoassay supports specialized testing beyond standard clinical use. Reliable signal detection assists scientific teams in exploring new biomarkers, advancing innovation across diagnostic fields that connect laboratory findings with practical healthcare advancement.
|
Forecast Period |
2025-2032 |
|
Market Size in 2025 |
$12.9 Billion |
|
Market Size by 2032 |
$17.8 Billion |
|
Growth Rate from 2025 to 2032 |
4.7% |
|
Base Year |
2024 |
|
Regions Covered |
North America, Europe, Asia-Pacific, South America, Middle East & Africa |
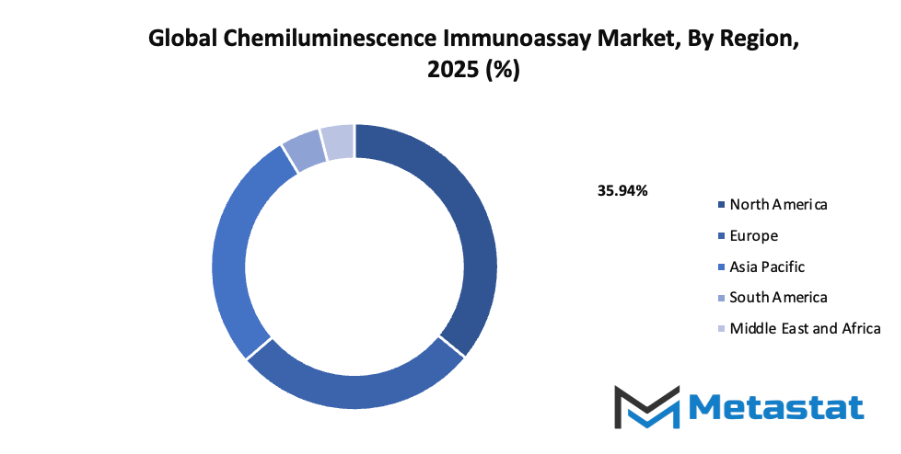
By Region:
- Based on geography, the global chemiluminescence immunoassay market is divided into North America, Europe, Asia-Pacific, South America, and the Middle East & Africa.
- North America is further divided into the U.S., Canada, and Mexico, whereas Europe consists of the UK, Germany, France, Italy, and the Rest of Europe.
- Asia-Pacific is segmented into India, China, Japan, South Korea, and the Rest of Asia-Pacific.
- The South America region includes Brazil, Argentina, and the Rest of South America, while the Middle East & Africa is categorized into GCC Countries, Egypt, South Africa, and the Rest of the Middle East & Africa.
Growth Drivers
- Growing demand for high-sensitivity and rapid diagnostic testing:
Growing demand for high-sensitivity and rapid diagnostic testing encourages wider adoption of advanced immunoassay systems. Faster detection supports earlier treatment decisions, reducing delays in patient care. Many healthcare facilities now favour tests that deliver results quickly without sacrificing accuracy. Higher throughput and reliable data encourage growing interest in the global chemiluminescence immunoassay market. - Increasing prevalence of chronic and infectious diseases requiring accurate detection:
Higher numbers of chronic and infectious diseases encourage laboratories to select methods that allow accurate detection during early stages. Precise biomarker measurement supports timely therapy and better patient outcomes. Increasing awareness of preventive healthcare encourages routine screenings, supporting additional demand across hospitals and diagnostic centers.
Challenges and Opportunities
- High cost of equipment and reagents:
Advanced analyzers, maintenance needs, and specialized reagents raise total laboratory expenses. Smaller facilities may hesitate due to heavy investment requirements, limiting broader adoption. Budget restrictions in developing regions slow expansion, even when advanced testing would improve healthcare outcomes. Cost reduction strategies could support more widespread purchasing decisions. - Requirement for skilled personnel to operate and interpret results:
Operation of chemiluminescence analyzers requires trained laboratory professionals, along with strong understanding of calibration, maintenance, and result interpretation. Limited availability of trained staff can delay installation and reduce testing accuracy. Continuous training programs may reduce errors and improve confidence in diagnostic results.
Opportunities
- Integration with automated and point-of-care testing systems for faster and decentralized diagnostics:
Integration with automated platforms and point-of-care solutions offers faster workflows and testing flexibility in clinics, emergency rooms, and outreach programs. Reduced manual steps lower chances of human error and improve consistency in reporting. Increased convenience supports broader adoption and enhances diagnostic accessibility across multiple healthcare environments.
Competitive Landscape & Strategic Insights
The global chemiluminescence immunoassay market reflects strong competition and steady progress. Major participation from recognized international corporations and fast-growing regional suppliers creates an environment where new ideas and improvement happen on a regular basis. Large corporations such as Abbott Laboratories, Beckman Coulter Inc., DiaSorin S.p.A., Shenzhen Mindray Bio-Medical Electronics Co., Ltd., F. Hoffmann-La Roche AG, Siemens Healthineers, and Ortho Clinical Diagnostics provide advanced testing systems along with reliable production capacity. Smaller suppliers add fresh methods, quicker adjustments to changing medical needs, and cost-efficient equipment for laboratories across many regions. Together, these forces encourage development of testing systems that support accurate diagnosis in hospitals, clinics, and research facilities.
Demand for high-quality diagnostic testing continues to grow. Hospitals, medical laboratories, and research centers look for technology that offers strong accuracy, shorter waiting time, and dependable results. Chemiluminescence Immunoassay technology answers those needs by using specialized reactions to detect different medical markers. Medical staff benefit from tools that support early disease detection and treatment planning. Faster and more precise diagnostics help health organizations reduce waiting lists and improve patient care. As healthcare systems expand, more organizations invest in advanced diagnostic platforms, driving higher demand for Chemiluminescence Immunoassay equipment.
Well-known corporations remain focused on long-term reliability, safe production procedures, and strong distribution structure. Regional suppliers often introduce devices with flexible pricing and local support, helping smaller hospitals gain access to modern diagnostic tools. Continuous improvements in automation, software guidance, and reagent quality support increased output and reduced errors inside laboratory settings. Each advancement encourages stronger confidence in testing outcomes.
Market progress depends on consistent innovation, thorough quality control, and dependable service. Investment in research encourages better sensitivity, smoother workflows, and user-friendly designs. As more healthcare centers adopt advanced testing platforms, manufacturers concentrate on stronger maintenance programs and simplified device management.
Market size is forecast to rise from USD 12.9 billion in 2025 to over USD 17.8 billion by 2032. Chemiluminescence Immunoassay will maintain dominance but face growing competition from emerging formats.
Competition from industry leaders and ambitious regional manufacturers strengthens the global chemiluminescence immunoassay market, leading to steady technological progress and greater access to reliable diagnostic solutions for healthcare providers around the world.
Report Coverage
This research report categorizes the global chemiluminescence immunoassay market based on various segments and regions, forecasts revenue growth, and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the global chemiluminescence immunoassay market. Recent market developments and competitive strategies such as expansion, type launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the global chemiluminescence immunoassay market.
Chemiluminescence Immunoassay Market Key Segments:
By Product Type
- Instruments
- Consumables
By Technology
- Chemiluminescence Enzyme Technology (CLEIA)
- Electrochemiluminescence immunoassay (ECLI)
- Microparticle Chemiluminescence Immunoassay
By Sample Type
- Blood
- Urine
- Saliva
- Other Sample Types
By Application
- Infectious Disease
- Endocrinology
- Oncology
- Cardiology
- Allergy Diagnostics
- Other Applications
Key Global Chemiluminescence Immunoassay Industry Players
- Abbott Laboratories
- Beckman Coulter Inc.
- DiaSorin S.p.A.
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- F. Hoffmann-La Roche AG
- Siemens Healthineers
- Ortho Clinical Diagnostics
WHAT REPORT PROVIDES
- Full in-depth analysis of the parent Industry
- Important changes in market and its dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional growth potential





 US: +1 3023308252
US: +1 3023308252






